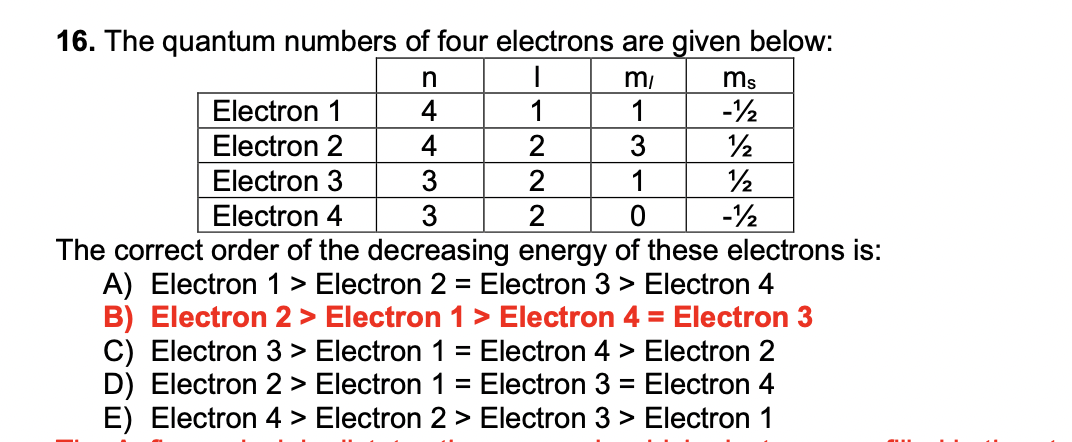
The quantum number of four electrons are given below
The quantum number of four electrons are given below: I.
Submitted by Anthony M. Solved by verified expert. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. The four quantum numbers of four electrons are given below.
The quantum number of four electrons are given below
Learn from their 1-to-1 discussion with Filo tutors. Total classes on Filo by this tutor - 22, Views: 5, Views: 6, Connect with our Chemistry tutors online and get step by step solution of this question. Are you ready to take control of your learning? Class Structure of Atom. The quantum number of four electrons are given below:. Solving time: 3 mins. Views: 5, students. Updated on: Mar 31, Exam: JEE Mains
Try Numerade free for 7 days View This Answer.
The higher the value of n, the higher the energy of the orbital. Last updated on Nov 2, Get Started. This question was previously asked in. Start Now. Calculation: I. Interested Candidates can submit online applications from 1st November to 30th November
The goal of this section is to understand the electron orbitals location of electrons in atoms , their different energies, and other properties. The use of quantum theory provides the best understanding to these topics. This knowledge is a precursor to chemical bonding. As was described previously, electrons in atoms can exist only on discrete energy levels but not between them. It is said that the energy of an electron in an atom is quantized, that is, it can be equal only to certain specific values and can jump from one energy level to another but not transition smoothly or stay between these levels. Generally speaking, the energy of an electron in an atom is greater for greater values of n. This number, n , is referred to as the principal quantum number.
The quantum number of four electrons are given below
Quantum numbers tell us the energy level, the number and the type of orbitals, and the spin of the electron. Collectively, they all describe the electron configurations. So, one can visualize the information conveyed by quantum numbers getting more specific as we go from the principal quantum number to the spin quantum number:. What orbitals a given atom has, and in which ones the electrons are located, depends on the energy level of the atom. Remember, the energy level of the atom is given by the principal quantum number, n which can easily be determined based on the period row the atom is located in the periodical table. This is what we discussed about the Bohr model of the hydrogen atom. There are orbits with fixed radii each associated with discrete energy, and this is described by the principal quantum number n. Remember, there are four types of atomic orbitals — s , p , d , and f. Each orbital has a characteristic shape shown below:.
Weatherhead pipe fittings
Log In. For people Speed of sound is highest in which medium? Please add your first playlist. Answer is not helpful. Step-by-step Solved, Expert Educator: The four quantum numbers of four electrons are given below. The maximum number of atomic orbitals associated with a principal quantum number 5 is. Question 3. Subtopic: Hydrogen Spectra. And from M plus L.
Forgot password? New user?
And similarly we can apply the other case also according to the office principle, the electrons are filled up in increasing order of the energy in this option. Calculus: Early Transcenden Topic: Chemical Bonding. Video Answer. State which of the following sets of quantum number would be possible and which would not be permisible for an electron in an atom. Subtopic: Electromagnetic Radiation. What is another name of quick lime? Please add your first playlist. In which of the following, energy of 2s orbital is minimum How many images will be formed if angle between mirrors is 30 degree? Share Question Copy Link. Go Premium and unlock limitless education potential beyond daily practice limits!

0 thoughts on “The quantum number of four electrons are given below”