Valency of atoms 1 to 20
We all know that the chemical formula for water harpyai H 2 O. What is the reason for this particular formula? Let us know more about Valency and how it helps in determining a formula!
Valency can be defined as combining the power of an element or radical. The valency chart consists of the list of valencies of the element. We know the chemical formula of salt and water is NaCl and H 2 O respectively. But have you ever thought about what the reason for this specific formula is? How are these derived?
Valency of atoms 1 to 20
You may assume that the valences of the elements—the number of electrons with which an atom will bond or form—are those that can be derived by looking at the groups columns of the periodic table. While these are the most common valences, the real behavior of electrons is less simple. Here is a table of element valences. Remember that an element's electron cloud will become more stable by filling, emptying, or half-filling the shell. Also, shells don't stack neatly one on top of another, so don't always assume an element's valence is determined by the number of electrons in its outer shell. Use limited data to select advertising. Create profiles for personalised advertising. Use profiles to select personalised advertising. Create profiles to personalise content. Use profiles to select personalised content. Measure advertising performance. Measure content performance. Understand audiences through statistics or combinations of data from different sources. Develop and improve services.
Read More: Atomic Number of Elements.
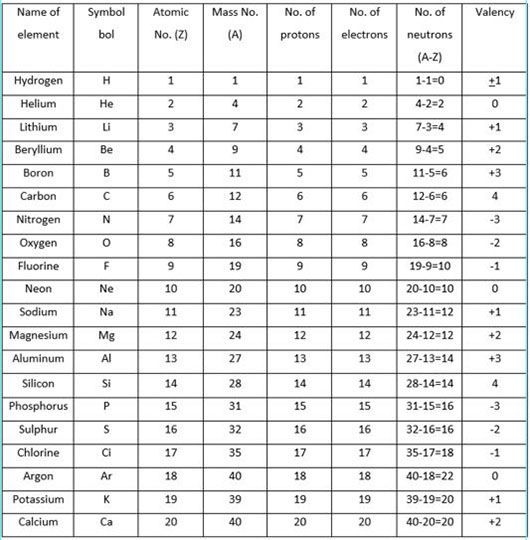
The valency of an element is a measure of its combining capacity and can be defined as. Oxidation State and valency are one of the most fundamental properties of elements and can be studied with the help of electron configurations. Electrons that are found in the outermost shell are generally known as valence electrons and the number of valence electrons determines the valency or valence of an atom. The general oxidation state of the elements of the periodic table is illustrated in the chart provided below. The valency of the first 30 elements of the periodic table is given below. While moving left to right across a period, the number of valence electrons of elements increases and varies between 1 to 8.
You may assume that the valences of the elements—the number of electrons with which an atom will bond or form—are those that can be derived by looking at the groups columns of the periodic table. While these are the most common valences, the real behavior of electrons is less simple. Here is a table of element valences. Remember that an element's electron cloud will become more stable by filling, emptying, or half-filling the shell. Also, shells don't stack neatly one on top of another, so don't always assume an element's valence is determined by the number of electrons in its outer shell. Use limited data to select advertising.
Valency of atoms 1 to 20
The valency of an element is a measure of its combining capacity and can be defined as. Oxidation State and valency are one of the most fundamental properties of elements and can be studied with the help of electron configurations. Electrons that are found in the outermost shell are generally known as valence electrons and the number of valence electrons determines the valency or valence of an atom. The general oxidation state of the elements of the periodic table is illustrated in the chart provided below. The valency of the first 30 elements of the periodic table is given below. While moving left to right across a period, the number of valence electrons of elements increases and varies between 1 to 8. But the valency of elements, when combined with H or O first, increases from 1 to 4 and then it reduces to zero. Consider two compounds containing oxygen Na 2 O and F 2 O. In F 2 O, the electronegativity of F is more than oxygen.
Thekkady cafe
Develop and improve services. Atomic Number. Thus, it is the number of valence electron an atom has to gain or lose from its outermost orbit. Suggested Videos. So, the valency of nitrogen is 3. Stability is also determined by the ability of atoms to gain electrons. They have 4 electron in their outermost shell. This article is being improved by another user right now. Save Article. Therefore, the valency of hydrogen is one. While moving left to right across a period, the number of valence electrons of elements increases and varies between 1 to 8. However, the reactivity of other elements depends upon their capacity to gain noble gas configuration. We get all the necessary information related to valency through this article.
The Valency For All the Elements determines the number of electrons it gains, loses, or shares when forming chemical bonds. This paragraph will provide an overview of the valency for all the elements in the periodic table, highlighting the diversity of chemical interactions and the significance of valency in predicting compound formation. Valency is a fundamental concept in chemistry that describes the combining capacity or the number of bonds an element can form with other elements.
Mentor September 29, at am. The valence electrons take part in any chemical reaction because the outermost orbit usually contains more energy than the electrons present in other orbits. Share Share Share Call Us. What is carbon valency? Riya sayal August 5, at pm. What Is Catalyst. So, valency cannot be related to charge. Alok kumar September 28, at pm. The valency of an element is a measure of its combining capacity and can be defined as. Easy Normal Medium Hard Expert.

I think, that you are mistaken. Write to me in PM, we will talk.
I am assured, that you have misled.