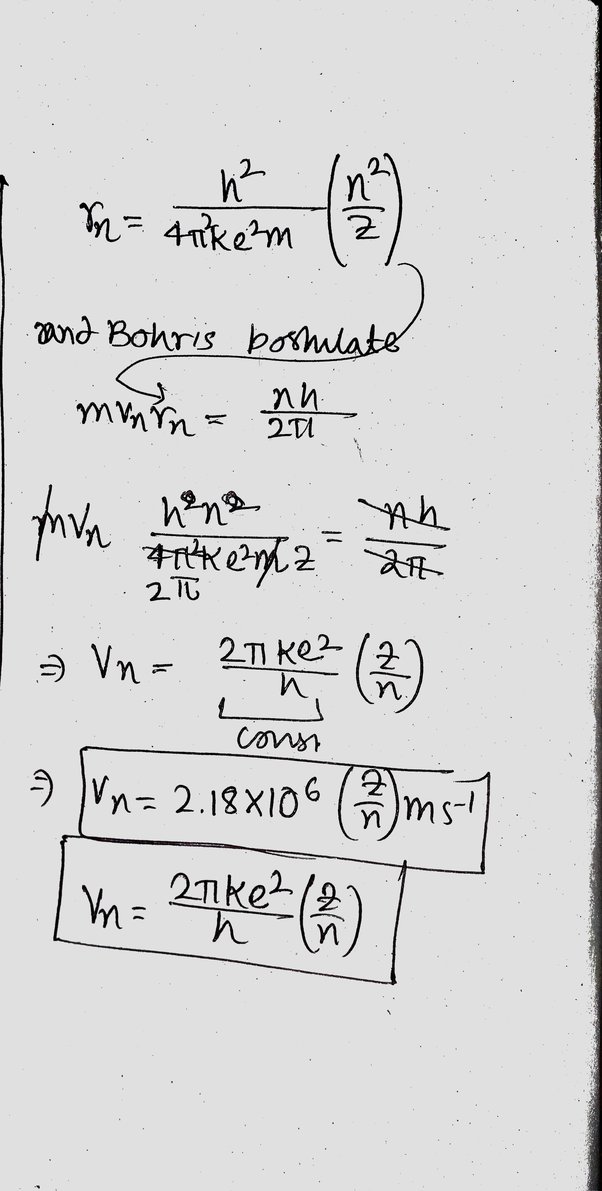
Velocity of an electron in nth orbit
From the above explanation, we can see that the speed on n th electron in a Hydrogen atom can be expressed as.
Angular momentum. Speed of moving electron in the orbit. Planck's constant. Dont't have an account? Register Now.
Velocity of an electron in nth orbit
The velocity of the electron in the first Bohr's orbit is 2. The linear velocity of electron in third orbit is. The velocity of electrons in the ground state of H- atom is 2. The velocity of an electron in the first Bohr orbit of hydrogen atom is 2. Its velocity in the second orbit would be. If the speed of electron in the first bohr orbit of hydrogen atom is x then the speed of the electron in the third Bohr orbit of hydrogen is. The velocity of an electron in the first orbit of H atom is v. The velocity of an electron in the first orbit of H-atom is v. The velocity of an electron in the 2nd orbit. The circumference of the first Bohr orbit in H atom is 3. What is the velocity of the electron of this orbit? The velocity of the electron in the first Bohr orbit of H-atoms is 2. If the speed of electron in the first bohr orbit of hydrogen atom is x
Colleges Colleges Accepting B. No Try it. What will be the velocity of the electron in the first orbit?
Submitted by Megan W. Solved by verified expert. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. The velocity of an electron in its fifth orbit will be. Calculate the speed of the electron in that orbit.
Hey there! We receieved your request. Various other concepts like radiation less orbits and stationary states were also introduced in the Bohr Model. We discuss the postulates of Bohr Atomic theory. Bohr introduced the concept of radiation less orbits in which the electrons revolve as usual around the nucleus but without radiating any kind of energy which is contrary to the laws of electromagnetism. This was a hypothesis, but at least a working one. Radiation occurred only when an electron made a transition from one stationary state to another. The difference between the energies of the two states was radiated as a single photon. Absorption occurred when a transition occurred from a lower stationary state to a higher stationary state. He also introduced the correspondence principle which states that the spectrum is continuous and the frequency of light emitted equals the frequency of the electron.
Velocity of an electron in nth orbit
Bohr's model of the atom was valuable in demonstrating how electrons were capable of absorbing and releasing energy, and how atomic emission spectra were created. However, the model did not really explain why electrons should exist only in fixed circular orbits, rather than being able to exist in a limitless number of orbits with different energies. In order to explain why atomic energy states are quantized, scientists had to rethink their views of the nature of the electron and its movement. Planck's investigation of the emission spectra of hot objects and the subsequent studies into the photoelectric effect had proven that light was capable of behaving both as a wave and as a particle.
Hot milfs fuck
Text Solution. In Bohr's orbit, kinetic energy of an electron in the n t h orbit of an atom in terms of angular momentum is. In how many years will it become 16 times itself? Phone Number. Sign Up. The radius of germanium Ge nuclide is measured to be twice the radiu An electron jumps from the 3rd orbit to the ground orbit in the hydrog The velocity of an electron in the first Bohr orbit of hydrogen atom is 2. This problem has been solved! Post Answer. Get Better Grades Now. Sign up Login. Notes Access past notes and exams matches to your classes Study Groups Study with your friends by joining virtual study sessions Free Unlocks Download the mobile app and receive 3 free video solutions. Quick links BTech M.
The model has a special place in the history of physics because it introduced an early quantum theory, which brought about new developments in scientific thought and later culminated in the development of quantum mechanics. When we use a prism to analyze white light coming from the sun, several dark lines in the solar spectrum are observed Figure 6.
The velocity of an electron in the first Bohr orbit of hydrogen atom i Competition Change. If the speed of electron in the first bohr orbit of hydrogen atom is x then the speed of the electron in the third Bohr orbit of hydrogen is. Sign up Login. Ace Chat Your personal AI tutor, companion, and study partner. The relation between force and acceleration of a body is given by-. Kinetic energy of electron in n th orbit in H-atom. Chemistry Nimbus Learning. The electron in the nth orbit of hydrogen atom has a radius:. The velocity of electrons in the ground state of H- atom is 2. Hospitality and Tourism Change. Get Better Grades Now. Learn today!

I congratulate, what necessary words..., a brilliant idea