What is formula of bleaching powder
It exists as an important raw material for the production of various chemical compounds such as caustic soda sodium hydroxidebleaching powder Ca OCl 2etc.
The chemical formula of bleaching powder or formula for Calcium hypochlorite will be explained right here along with the molecular structure that it has. It is important to note that the component Calcium hypochlorite is the main ingredient that is a part of the bleaching powder. Also, the main use of bleaching powder is for cleaning purposes and much more. The bleaching powder formula states that it is an inorganic compound by nature. Calcium hypochlorite and bleaching powder are both often synonymously in use. Most students would want to know about the molecular chemistry formula of bleaching powder.
What is formula of bleaching powder
Calcium hypochlorite is an inorganic compound with formula Ca ClO 2. It is a white solid, although commercial samples appear yellow. It strongly smells of chlorine, owing to its slow decomposition in moist air. This compound is relatively stable as a solid and solution and has greater available chlorine than sodium hypochlorite. Charles Tennant and Charles Macintosh developed an industrial process in the late 18th century for the manufacture of chloride of lime. Calcium hypochlorite is commonly used to sanitize public swimming pools and disinfect drinking water. In solution, calcium hypochlorite could be used as a general purpose sanitizer, [5] but due to calcium residue making the water harder , sodium hypochlorite bleach is usually preferred. Calcium hypochlorite is a general oxidizing agent and therefore finds some use in organic chemistry. Calcium hypochlorite is produced industrially by treating moist slaked lime Ca OH 2 with chlorine. The one-step reaction is shown below: [3]. Industrial setups allow for the reaction to be conducted in stages to give various compositions, each producing different ratios of calcium hypochlorite, unconverted lime, and calcium chloride.
EPFO Assistant.
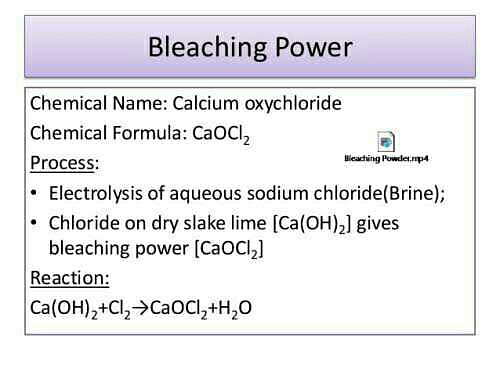
The chemical formula of bleaching powder is Ca OCl 2. Bleaching powder is synthesized by the action of chlorine gas produced from the chlor-alkali process on dry slaked lime Ca OH 2. The chemical equation is as follows:. Byju's Answer. The chemical formula of bleaching powder is…….
The chemical formula of bleaching powder or formula for Calcium hypochlorite will be explained right here along with the molecular structure that it has. It is important to note that the component Calcium hypochlorite is the main ingredient that is a part of the bleaching powder. Also, the main use of bleaching powder is for cleaning purposes and much more. The bleaching powder formula states that it is an inorganic compound by nature. Calcium hypochlorite and bleaching powder are both often synonymously in use. Most students would want to know about the molecular chemistry formula of bleaching powder. So, here we are going to discuss that right now.
What is formula of bleaching powder
It exists as an important raw material for the production of various chemical compounds such as caustic soda sodium hydroxide , bleaching powder Ca OCl 2 , etc. Bleaching powder is synthesized by the action of chlorine gas produced from the chlor-alkali process on dry slaked lime Ca OCl 2. Sodium hydroxide crystal 3D ball structure. It is prepared on dry slaked lime with chlorine gas. Bleaching powder is basic in character. It gives calcium chloride, chlorine and water when bleaching powder reacts with hydrochloric acid. Sodium hydroxide NaOH is a metallic caustic base, also known as lye or caustic soda. An alkaline, caustic soda is widely used in many industries, mostly in the manufacture of pulp and paper, textiles, drinking water and detergents as a strong chemical base.
Stuffing for stuffed animals
MP Mahila Supervisor. Eugenics is the study of:. MP Staff Nurse. Never mix ammonia with bleaching powder as their reaction will create a deadly gas that can cause breathing problems. RPSC 1st Grade. Goa Police Constable. Tripura TET. Which of the following molecules has zero dipole moment? Sanitary and Waste Mgmt. Territorial Army. Solved examples of Bleaching Powder Here are a few solved examples of Bleaching powder: Problem1: How many moles of Bleaching powder can be produced if 10 moles of Chlorine react with 10 moles of Calcium Hydroxide? NVS Lab Attendant.
Bleaching powder, also known as calcium hypochlorite, is a white or grayish-white powder that is widely used as a disinfectant and bleaching agent. Bleaching power has many uses, including as a bleaching agent, disinfectant and sanitizer.
Bihar Panchayati Raj Clerk. Bleaching powder is one of the most powerful oxidising agents used in different industries. DDA Patwari. S2CID Keep bleaching powder away from your eyes and skin as it causes irritation and burns. Bleaching powder has a pale yellowish colour. Punjab Police Jail Warder. Oil India Senior Officer. Manipur PSC. Table of Content. Which of the following substances is not an aromatic compound? Kerala PSC.

I can not participate now in discussion - it is very occupied. But I will return - I will necessarily write that I think.
It was specially registered at a forum to tell to you thanks for the help in this question how I can thank you?