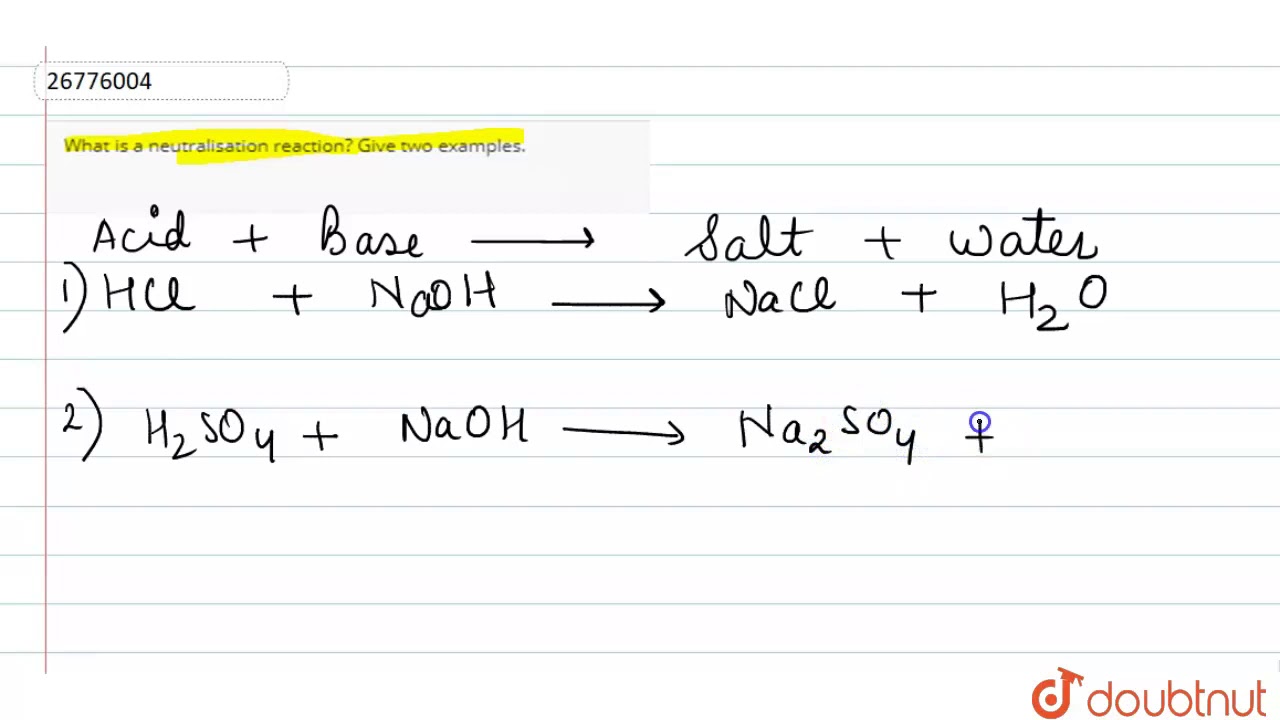
What is neutralisation reaction give two examples
Last updated at May 29, by Teachoo.
This is slightly incorrect, but until additional concepts were developed, a better definition needed to wait. The chemical opposite of an acid is a base. These original definitions were proposed by Arrhenius the same person who proposed ion dissociation in , so they are referred to as the Arrhenius definition of an acid and a base, respectively. Do we really have bare protons moving about in aqueous solution? The reaction of an acid and a base is called a neutralization reaction. In fact, the general reaction between an acid and a base is. In chemistry, the word salt refers to more than just table salt.
What is neutralisation reaction give two examples
.
Since acid and base neutralize each other's effect,it is called neutralization reaction. Write a balanced chemical equation for the neutralization reaction between each given acid and base.
.
The neutralization of a strong acid and strong base has a pH equal to 7. The neutralization of a strong acid and weak base will have a pH of less than 7, and conversely, the resulting pH when a strong base neutralizes a weak acid will be greater than 7. When a solution is neutralized, it means that salts are formed from equal weights of acid and base. Because salts are formed from neutralization reactions with equivalent concentrations of weights of acids and bases: N parts of acid will always neutralize N parts of base. This can be written in terms of the ions and canceled accordingly. When a strong acid and a strong base fully neutralize, the pH is neutral. Neutral pH means that the pH is equal to 7. When a strong acid completely neutralizes a strong base, the pH of the salt solution will always be 7. A weak acid, weak base reaction can be shown by the net ionic equation example:. The equivalence point of a neutralization reaction is when both the acid and the base in the reaction have been completely consumed and neither of them are in excess.
What is neutralisation reaction give two examples
This definition is based on the behavior of acids and bases when they are added to water. First we need to look at the autoionization of water. When two water molecules bump into each other a proton can be transferred from one to the other forming hydronium and hydroxide ions eq. These are compounds that increase the hydroxide concentration. They can be soluble ionic compounds that have hydroxide ions, or compounds that remove a proton from water forming hydroxide, and the later can be molecules.
Weather henrico va 23233
Please login to view more pages. Since acid and base neutralize each other's effect,it is called neutralization reaction. What is the net ionic equation between these two substances if the salt formed is insoluble? Write a balanced chemical equation for each neutralization reaction in Exercise 3. Join Teachoo Black. The chloride ions are the only spectator ions here, so the net ionic equation is. Complete ionic equation:. The chemical opposite of an acid is a base. Old search 1. Answer A reaction of an acid with a base to form salt and water is a neutralization reaction.
What is a neutralisation reaction?
The difference is simply the presence of an extra water molecule as a product. Join Teachoo Black. Washing with acids like HCl is one way to remove rust and rust stains, but HCl must be used with caution! Next: Oxidation-Reduction Reactions. To balance the equation, we need to realize that there will be two H 2 O molecules, so two HNO 3 molecules are required:. A reaction of an acid with a base to form salt and water is a neutralization reaction. Neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. Answer A reaction of an acid with a base to form salt and water is a neutralization reaction. Your browser does not support the audio element. Learn in your speed, with individual attention - Teachoo Maths 1-on-1 Class. To help Teachoo create more content, and view the ad-free version of Teachooo Maths Classes.

What is it to you to a head has come?
It is obvious, you were not mistaken
In my opinion you are mistaken. I can prove it. Write to me in PM.