Xeo2f2 lewis structure
N will be equal to the number of monovalent fluorine atoms bonded to the central atom. C will be the charge of cation and A will xeo2f2 lewis structure the charge of anion.
Laurence Lavelle Skip to content. Quick links. Email Link. XeO2F2 Lewis Structure Post by Bryce Ramirez 1J » Wed Nov 20, am Why does Xenon have two double bonds with oxygen and 2 single bonds with fluorine and a lone pair to make the lowest energy level for its lewis structure. I thought that you are only allowed to assign 8 electrons to each atom, but if you were to add it up, Xe would have 14 electrons. Not sure if this was the correct lewis structure I saw or if there is a lower energy structure, but on other problems I have also seen the central atom being assigned more than 8 electrons and have been confused why this is allowed.
Xeo2f2 lewis structure
In order to find the total valence electrons in XeO2F2 molecule , first of all you should know the valence electrons present in xenon atom, oxygen atom as well as fluorine atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Xenon is a group 18 element on the periodic table. Oxygen is group 16 element on the periodic table. Fluorine is group 17 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. You can see the electronegativity values of xenon atom Xe , oxygen atom O and fluorine atom F in the above periodic table. If we compare the electronegativity values of xenon Xe , oxygen O and fluorine F then the xenon atom is less electronegative. Now in the XeO2F2 molecule, you have to put the electron pairs between the xenon-oxygen atoms and xenon-fluorine atoms. This indicates that these atoms are chemically bonded with each other in a XeO2F2 molecule. Here in the sketch of XeO2F2 molecule, you can see that the outer atoms are oxygen atoms O and fluorine atoms F.
Did not receive OTP? Jump to.
Transcript: This is Dr. Let's do the XeO2F2 Lewis structure. For XeO2F2, we have a total of 34 valence electrons. We'll put the Xe in the center and then we'll put an Oxygen here and here, and a Fluorine right there and there. We'll put a chemical bond right here between the atoms. Each bond represents two electrons, so we've used 2, 4, 6, 8 valence electrons.
XeO 2 F 2 xenon dioxydifluoride has one xenon atom, two oxygen atoms, and two fluorine atoms. In the XeO 2 F 2 Lewis structure, there are two single bonds and two double bonds around the xenon atom, with two fluorine atoms and two oxygen atoms attached to it. Each fluorine atom has three lone pairs, each oxygen atom has two lone pairs, and the xenon atom has one lone pair. In the periodic table , xenon lies in group 18, oxygen lies in group 16, and fluorine lies in group Hence, xenon has eight valence electrons, oxygen has six valence electrons, and fluorine has seven valence electrons.
Xeo2f2 lewis structure
In this article, we will discuss about xeo2f2 lewis structure, hybridization, formal charge, and its geometry. Xenon dioxide difluoride, sometimes known as XeO 2 F 2 , is an inorganic molecule with the chemical formula XeO 2 F 2. One xenon atom, two oxygen atoms, and two fluorine atoms make up XeO 2 F 2 xenon dioxydifluoride. Two single bonds and two double bonds surround the xenon atom in the Lewis structure of XeO2F2, which is surrounded by two fluorine atoms and two oxygen atoms. There are three lone pairs in each fluorine atom, two lone pairs in each oxygen atom, and one lone pair in each xenon atom. The Lewis structure of an atom is a simplified depiction of the nucleus and valence electrons in its atomic structure.
Fridays restaurant reviews
We get 5 as the final number which further suggests sp 3 d hybridization. Xenon has 4 bond pairs and 1 lone pair. If we compare the electronegativity values of xenon Xe , oxygen O and fluorine F then the xenon atom is less electronegative. As for the angles, the O—Xe—O angle will be So while all the charges add up to zero, overall we'd like to have everything, each of the atoms, at zero itself. Now in the XeO2F2 molecule, you have to put the electron pairs between the xenon-oxygen atoms and xenon-fluorine atoms. XeO2F2 has a total of 34 electrons, when you draw the lewis structure and add at least one bond between xenon and the other atoms xenon is in the center because it is least electronegative you find that the number of electrons used is Scroll to Top. Your Mobile number and Email id will not be published. N will be equal to the number of monovalent fluorine atoms bonded to the central atom. See the Big List of Lewis Structures. Valence electrons are the electrons that are present in the outermost orbit of any atom. When we recalculate the formal charges, we can see they're now all zero.
Ready to learn how to draw the lewis structure of XeO2F2? Here, I have explained 5 simple steps to draw the lewis dot structure of XeO2F2 along with images. The Xenon atom has 1 lone pair.
XeO2F2 Lewis Structure Post by Bryce Ramirez 1J » Wed Nov 20, am Why does Xenon have two double bonds with oxygen and 2 single bonds with fluorine and a lone pair to make the lowest energy level for its lewis structure. The whole sum will be divided by 2 at the end. In order to find the total valence electrons in XeO2F2 molecule , first of all you should know the valence electrons present in xenon atom, oxygen atom as well as fluorine atom. I thought that you are only allowed to assign 8 electrons to each atom, but if you were to add it up, Xe would have 14 electrons. In Xenon Dioxide Difluoride, there will be 5 sp 3 d hybrid orbitals. Valence electrons are the electrons that are present in the outermost orbit of any atom. Your Mobile number and Email id will not be published. The repulsion between bond pair and lone pair of electrons will be more. Your email address will not be published. So you have seen the above image by now, right? We have to use 34 electrons in our diagram. If we compare the electronegativity values of xenon Xe , oxygen O and fluorine F then the xenon atom is less electronegative. Put your understanding of this concept to test by answering a few MCQs.

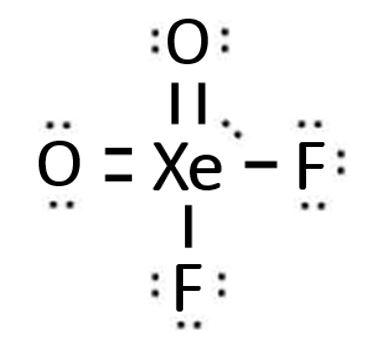
Yes you the talented person