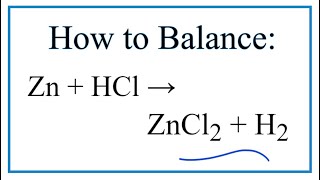
Zinc plus hydrochloric acid
Wiki User. A word equation represent the reactions between metals and acids.
Wiki User. A word equation represent the reactions between metals and acids. The reaction for zinc and hydrochloric acid would be, zinc plus hydrochloric acid produces hydrogen plus zinc chloride. Zinc plus hydrochloric acid produces zinc chloride plus hydrogen gas. Common one.
Zinc plus hydrochloric acid
.
Nitric acid plus zinc oxideNitricoxide. Distillation, evaporation, filtration or neutralization?
.
We can use a gas syringe to measure the reaction of metals with dilute acid. When zinc reacts with hydrochloric acid it produces zinc chloride and hydrogen gas. We can measure the rate of the reaction by measuring how fast the reaction produces hydrogen. This requires a conical flask and gas syringe. Similarly, when calcium carbonate reacts with dilute hydrochloric acid, it produces carbon dioxide gas. We can measure the rate of the reaction by measuring how fast the reaction produces carbon dioxide. Hydrogen peroxide decomposes in the presence of a catalyst close catalyst A substance that increases the rate of a chemical reaction without being used up. We can measure how each catalyst affects the rate of the reaction by measuring how fast it produces oxygen.
Zinc plus hydrochloric acid
The hydrogen causes bubbling during the reaction, and can be detected using a burning splint which produces a squeaky pop sound. In general, the more reactive the metal, the faster the reaction. This is indicated by more bubbles being given off per second from the metals with higher reactivity, as shown by this diagram. The diagram shows that aluminium is the most reactive of the four metals, followed by zinc, then iron and finally, copper. Note that the reaction between a metal hydroxide and an acid can be represented by an ionic equation between the hydrogen ions and the hydroxide ions to form water molecules. Also note that the reaction of metal hydroxides with acids is exothermic ie heat energy is given out. Also note that the reaction of metal oxides with acids is exothermic ie heat energy is given out. When acids react with carbonates, such as calcium carbonate found in chalk, limestone and marble , a salt, water and carbon dioxide are made. The carbon dioxide causes bubbling during the reaction, which is observed as fizzing.
Town synonym
What is the equation for the reaction of benzoic acid and zinc oxide? What is the reaction for metal plus acid - salt plus hydrogen? What is the balance equation for sulphuric acid with zinc? The products are zinc chloride and hydrogen. If it isn't, then sorry. The reaction for zinc and hydrochloric acid would be, zinc plus hydrochloric acid produces hydrogen plus zinc chloride. Best Answer. What is the Word equation for sulfuric acid? What is the symbol equation for zinc plus hydrochloric acid zinc plus chloride hydrogen? Still have questions? What is the equation for the dissociation of hydrochloric acid? What is the equation for zinc plus sulfuric acid? Find more answers. Approximately g of hydrogen gas are produced when Tags Metal and Alloys Subjects.
It is fairly obvious that zinc metal reacts with aqueous hydrochloric acid!
Log in. What is the balanced chemical rquation of zinc metal plus hydrochloric acid zinc chloride solution plus hydrogen? What is the equation for the dissociation of hydrochloric acid? Zinc chloride and hydrogen gas are formed in the following reaction. A word equation represent the reactions between metals and acids. What is the symbol equation for zinc plus hydrochloric acid zinc plus chloride hydrogen? Wiki User. What is the word equation for hydrochloric acid and zinc carbonate? The material on this site can not be reproduced, distributed, transmitted, cached or otherwise used, except with prior written permission of Answers. Study now See answers 9. All Rights Reserved. Zinc plus hydrochloric acid produces zinc chloride and hydrogen gas. Still have questions? Matter can neither be created nor destroyed. In which of the following does a chemical change take place?

I consider, that you are mistaken. I can defend the position. Write to me in PM.
The matchless message, is pleasant to me :)
You are not right. I am assured. I suggest it to discuss. Write to me in PM, we will communicate.