9 bbn structure
From Wikimedia Commons, the free media repository. File information.
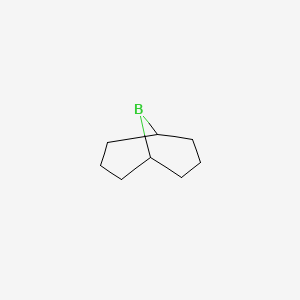
This colourless solid is used in organic chemistry as a hydroboration reagent. The compound exists as a hydride-bridged dimer, which easily cleaves in the presence of reducible substrates. The compound is commercially available as a solution in tetrahydrofuran and as a solid. Its highly regioselective addition on alkenes allows the preparation of terminal alcohols by subsequent oxidative cleavage with H 2 O 2 in aqueous KOH. The steric demand of 9-BBN greatly suppresses the formation of the 2-substituted isomer compared to the use of borane. Contents move to sidebar hide.
9 bbn structure
.
The compound is commercially available as a solution in tetrahydrofuran and as a solid. MIME type. Other names Borabicyclononane Banana borane.
.
B -alkylBBN preparation is itself a two-step process, thus restricting the utility of the 9-BBN for synthetic purposes. These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves. This is a preview of subscription content, log in via an institution. Unable to display preview. Download preview PDF. Article Google Scholar. Google Scholar. CAS Google Scholar. Heyden, London.
9 bbn structure
The hydroboration-oxidation of alkynes is similar to the reaction with alkenes. However, there is one important difference. The alkyne has two pi bonds and both are capable of reacting with borane BH 3. To limit the reactivity to only one of the pi bonds within the alkyne, a dialkyl borane reagent R 2 BH is used.
Verizon sign in
Structured data Items portrayed in this file depicts. Public domain Public domain false false. The steric demand of 9-BBN greatly suppresses the formation of the 2-substituted isomer compared to the use of borane. The source code of this SVG is invalid due to 13 errors. Tools Tools. EC Number. Chemical compound. The following other wikis use this file: Usage on de. Download as PDF Printable version. You cannot overwrite this file. The compound is commercially available as a solution in tetrahydrofuran and as a solid. CAS Number.
An especially valuable group of intermediates can be prepared by addition of an compound to carbon-carbon double or triple bonds:. The reaction is called hydroboration and is a versatile synthesis of organoboron compounds.
CAS Number. Borabicyclononane Banana borane. Chemical formula. Organic Syntheses via Boranes. The compound exists as a hydride-bridged dimer, which easily cleaves in the presence of reducible substrates. Toggle limited content width. Precautionary statements. Description 9-BBN structure. The substitution of any brackets is due to technical restrictions. Public domain Public domain false false. This image of a simple structural formula is ineligible for copyright and therefore in the public domain , because it consists entirely of information that is common property and contains no original authorship. Namespaces File Discussion. Organic Syntheses ; Collected Volumes , vol.

In it something is also idea excellent, I support.
What charming idea