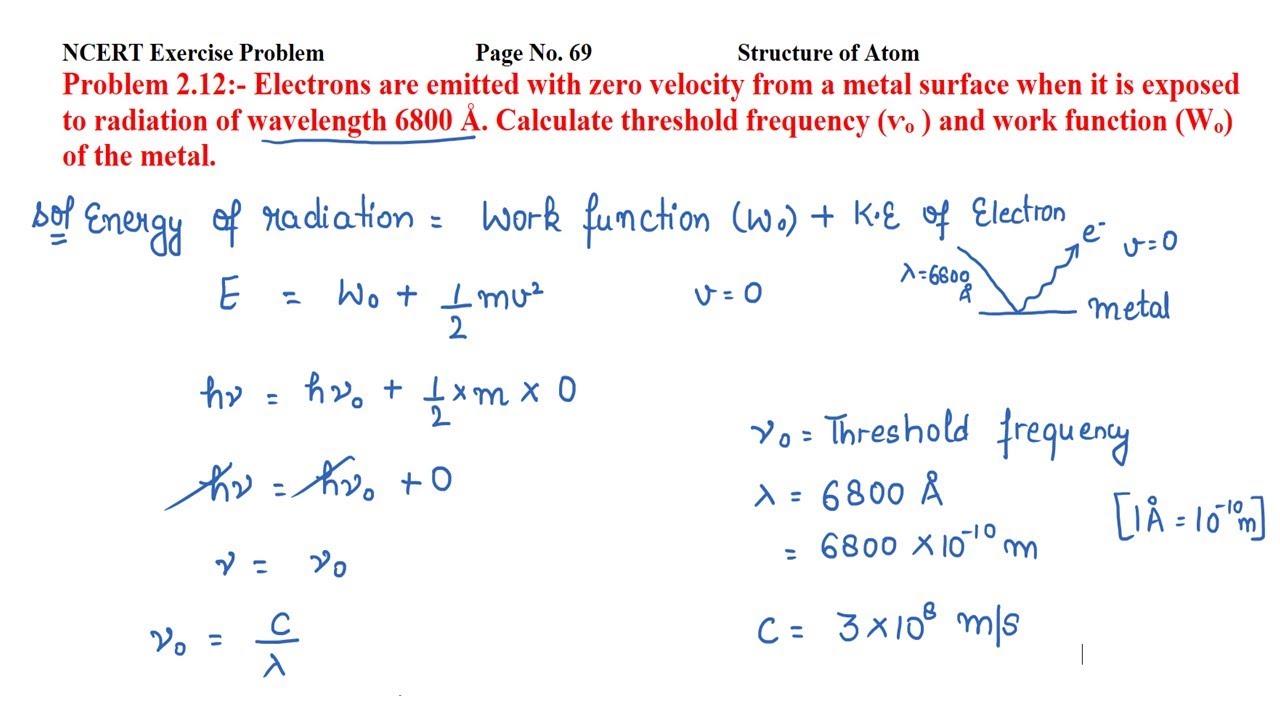
Electrons are emitted with zero velocity
Mention its one practical application in daily life.
Electrons are emitted from an electron gun at almost zero velocity and are accelerated by an electric field E through a distance of 1. The electrons are now scattered by an atomic hydrogen sample in ground state. What should be the minimum value of E so that red light of wavelength Byju's Answer. Open in App.
Electrons are emitted with zero velocity
Given the wavelength of radiation is. But the electrons are emitted with zero velocity from a metal surface when it is exposed to radiation. That means the kinetic energy will be zero. So, the Threshold frequency will be:. CBSE Class 12 physics board exam today; values of physical constants, weightage. Dont't have an account? Register Now. Colleges Colleges Accepting B. Quick links BTech M. Computer Application and IT Change.
Study Abroad Change. Calculate the wav
Mention its one practical application in daily life. Calculate thresholds freqency V o and work function W o of the mental. Calculate threshold frequency v 0 and work function W 0 of the metal. Calculate i threshold frequency ii work function. Will there be photoelectric emission or not? Determine the work function of the metal, the threshold wavelength of metal and the stopping potential difference required to stoop the emission of electrons. When a certain metal was irradiated with light of frequency 1.
Given the wavelength of radiation is. But the electrons are emitted with zero velocity from a metal surface when it is exposed to radiation. That means the kinetic energy will be zero. So, the Threshold frequency will be:. JEE Main session 2 exam city intimation slip expected by March last week. Dont't have an account? Register Now.
Electrons are emitted with zero velocity
Book Store. Download books and chapters from book store. Previous Year Papers. Currently only available for. Class 10 Class
Family guy peter long legs
Metal surface emits photoelectrons when light of wavelength nm is incident on it. Learn Change. Phone Number. How much energy will be released when a sodium ion and a chloride ion, The work function of the metal is. Colleges Colleges Accepting B. Welcome Back : To keep connected with us please login with your personal information by phone. Pharma M. Given the wavelength of radiation is. I am already a member. The electrons are now scattered by an atomic hydrogen sample in ground state. Animation and Design Change. What should be the minimum value of E so that red light of wavelength Determine the work function of the metal, the threshold wavelength of metal and the stopping potential difference required to stoop the emission of electrons. The area of cross section of the beam remains constant.
Being fermions , no two electrons can occupy the same quantum state , per the Pauli exclusion principle. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a longer de Broglie wavelength for a given energy. Electrons play an essential role in numerous physical phenomena, such as electricity , magnetism , chemistry , and thermal conductivity ; they also participate in gravitational , electromagnetic , and weak interactions.
Animation and Design Change. The ionisation energy of a H-like atom is 4R h a. Option: 1 1 A certain loan amounts, under compound interest, compounded annually earns an interest of Rs. What are the possible values of l and m? Through what potential difference should the electrons be accelerated so that third line of Lyman series be emitted? Video Solution. An electron is in one of the 3d orbitals. An electron of kinetic energy E 0 is scattered by an atomic hydrogen sample in ground state. Dont't have an account? When a certain metal was irradiated with light of frequency 1.

0 thoughts on “Electrons are emitted with zero velocity”