Sulfate lewis dot structure
Lewis dot structure of SO 4 2 - :. Lewis Dot Structure of NO 2 - :. Byju's Answer. Open in App.
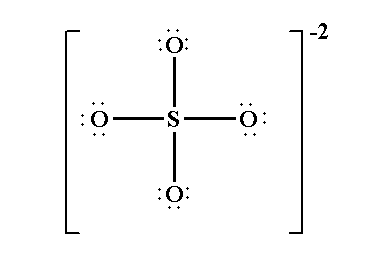
Lewis structure of sulfate ion is drawn in this tutorial step by step. Total valence electrons concept is used to draw the lewis structure of SO 4 In lewis structure of sulfate ion, there should be charges on several atoms due to -2 charge. Sulfate ion is one of the oxyanion of sulfur. Also, sulfate ion has a -2 charge. Sulfur atom is the center atom and four oxygen atoms are located around sulfur atom. There are no lone pairs in the last shell of sulfur atom.
Sulfate lewis dot structure
The sulfate ion Sulfate Standard is a polyatomic anion with the empirical formula SO 4 Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid, and many are prepared from that acid. The sulfate anion consists of a central sulfur atom surrounded by four equivalent oxygen atoms in a tetrahedral arrangement. The symmetry is the same as that of methane. First, determine the valence electron available for drawing the Lewis structure of SO 4 2- because the Lewis diagram represents valence electrons around atoms. Sulfur and oxygen atoms are located in the VIA group in the periodic table. So, oxygen and sulfur atoms have six electrons in their valence shell. Total electron pairs are determined by dividing the number of total valence electrons by two. For SO 4 2- ion, the Total pairs of electrons are In theory, the atom which is less electronegative remains at the center. Compare the electronegativity values of sulfur S and oxygen O ; the sulfur atom is less electronegative. Hence, the sulfur atom S is the center atom, and the oxygen atoms O are the outside atoms.
There are no electrons left from the valence electrons. Sulfur is the central atom and four oxygen atoms are located around the sulfur atom Adding electron pair between Sulfur and oxygen to represent a chemical bond. You may like Sodium bisulfate: uses, Produce method, and Regulation Mar 1, sulfate lewis dot structure, Is Sodium ferrocyanide safe as an anticaking agent?
.
Lewis structure of sulfate ion is drawn in this tutorial step by step. Total valence electrons concept is used to draw the lewis structure of SO 4 In lewis structure of sulfate ion, there should be charges on several atoms due to -2 charge. Sulfate ion is one of the oxyanion of sulfur. Also, sulfate ion has a -2 charge.
Sulfate lewis dot structure
Sulfate ion SO is one of the most common ions that people in chemistry need to deal with. This is a polyatomic anion having a negative charge of We can easily prepare sulfates via oxidizing metal sulfites and sulfides. We can also use sulfuric acid and metals to get our desired sulfate salts. Since we can easily get hold of this ion, be it naturally or synthetically, this helps us in our daily lives in a lot more ways than you can think of right now! From body and hygiene-care products like toothpaste, shampoos, soaps, and detergents to water treatment procedures, we can find the application of sulfate compounds everywhere.
Chi min restaurant
Total valence electrons concept is used to draw the lewis structure of SO 4 Sulfur atom is the center atom and four oxygen atoms are located around sulfur atom. There are no electrons left from the valence electrons. Maximum valnce of oxygen is two. Saturated and Unsaturated Hydrocarbon. Otherwise, we can think an oxygen atom of sulfate ion is replaced by a sulfur atom. Lewis Dot Structure of NO 2 - :. You see charges of atoms are reduced. Total electron pairs are determined by dividing the number total valence electrons by two. In addition, there is a -1 formal charge on the other two oxygen atoms. The uses and biological function of Octreotide. In new structure, charges of atoms are reduced than previous structure. How to draw Lewis dot structure of k3cro8. Lewis Structure Sulfate ion Lewis structure of sulfate ion is drawn in this tutorial step by step. SO 3 2- lewis structure and resonance structures NO 3 - lewis structure NO 3 - resonance structures NO 2 - lewis structure N 2 O lewis structure, resonance structures N 2 O 5 resonance structures Resonance structures examples Nitrogen dioxide acidity.
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms.
The sulfate ion Sulfate Standard is a polyatomic anion with the empirical formula SO 4 Now we are left with 14 valence electron Assigning the electrons such that the octet of nitrogen and oxygen is completed. This ensures a correct and stable Lewis representation for the sulfate SO 4 2- ion. We should minimize charges by converting lone pairs or pairs to bonds. Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Therefore, From the octet rule, we can draw the Lewis dot structures. Description The sulfate ion Sulfate Standard is a polyatomic anion with the empirical formula SO 4 Octreotide is a synthetic somatostatin analog with several palliative care indications, including managing ascites, bowel obstruction, diarrhoea, fistulae, and tumor secretions Feb 29, What is the charge of the metal ion in FeCl3? After marking electron pairs on atoms, we should mark the charges of each atom. Lewis Dot Structure of NO 2 - :. Total electron pairs are determined by dividing the number total valence electrons by two. When charges exist everywhere on atoms in the ion or molecule, that structure is not stable. So, convert one lone pair of electrons of one oxygen atom to make a new S-O bond. We should try to reduce charges on atoms as much as possible.

In my opinion it is obvious. I have found the answer to your question in google.com
Excuse for that I interfere � To me this situation is familiar. Let's discuss. Write here or in PM.
Quite right! It is excellent idea. It is ready to support you.