Example of ionic hydride
Hydride in Chemistry is the name of a compound containing Hydrogen Anion. Hydrides are chemical compounds with one atom of hydrogen and an extra atom.
Saline hydrides also known as ionic hydrides or pseudohalides are compounds form between hydrogen and the most active metals, especially with the alkali and alkaline-earth metals of group one and two elements. They bond with more electropositive metal atoms. Ionic hydrides are usually binary compounds i. They are all very reactive and readily react with various compounds. For example, when an alkali metal reacts with hydrogen gas under heat, an ionic hydride is produced. Alkali metal hydrides also react with water to produce hydrogen gas and a hydroxide salt:. Saline hydrides are formed by the Group 1 and 2 metals when heated with dihydrogen H 2.
Example of ionic hydride
Hydride , in simple terms, is said to be the anion of hydrogen. It is a chemical compound where the hydrogen atoms exhibit nucleophilic, basic or reducing properties. Compounds of hydrogen with less electronegative elements are known as hydrides. So, when hydrogen reacts with any other element, the product formed is considered to be a hydride. If we closely observe the periodic table, hydride formation is not seen from VA group elements, and this condition is known as the hydride gap. Hydrogen molecule usually reacts with many elements except noble gases to form hydrides. However, the properties may vary depending on the type of intermolecular force that exists between the elements, their molecular masses, temperature, and other factors. Hydrides are mainly divided into three major types or groups. The categories are decided based on what elements the hydrogen forms bonds with or simply on the basis of chemical bonding. The three types of hydrides are ionic, covalent, and metallic hydrides. We will learn about them in detail below. They are formed when hydrogen molecule reacts with highly electropositive s-block elements Alkali metals and alkaline earth metals. In solid-state, the ionic hydrides are crystalline, non-conducting and non-volatile.
They are stable compound and are boned by the metallic bonds. General Chemistry 9e.
The term hydride is commonly named after binary compounds that hydrogen forms with other elements of the periodic table. Hydride compounds in general form with almost any element, except a few noble gases. The trends and properties vary according to the type of intermolecular force that bonds the elements together, the temperature, its molecular masses, and other components. Hydrides are classified into three major groups, depending on what elements the hydrogen bonds to. The three major groups are covalent, ionic, and metallic hydrides.
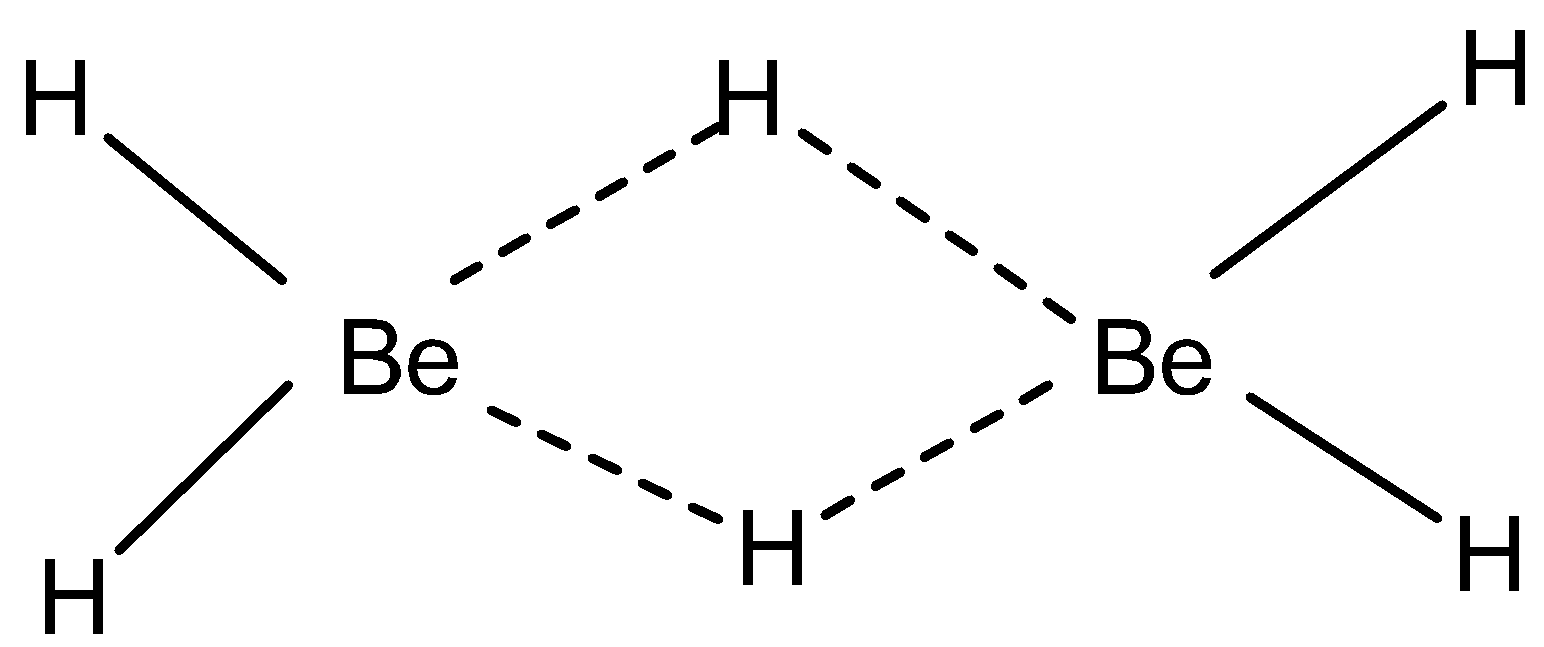
The combination of hydrogen with another element produces a hydride, E x H y. The formal charge or oxidation state of the hydrogen in these compounds is dependant on the relative electronegativity of the element in question. Hydrogen compounds with highly electropositive metals, i. As a consequence, in the solid state the hydride ion replicates that of a halide ion e. Unlike the halide ions that are soluble in water, the hydride ion reacts with water, 2. The liberation of hydrogen was used as a commercial source of hydrogen for small-scale applications. Group 1 and 2 metal hydrides can ignite in air, especially upon contact with water to release hydrogen, which is also flammable.
Example of ionic hydride
Hydride , in simple terms, is said to be the anion of hydrogen. It is a chemical compound where the hydrogen atoms exhibit nucleophilic, basic or reducing properties. Compounds of hydrogen with less electronegative elements are known as hydrides. So, when hydrogen reacts with any other element, the product formed is considered to be a hydride. If we closely observe the periodic table, hydride formation is not seen from VA group elements, and this condition is known as the hydride gap. Hydrogen molecule usually reacts with many elements except noble gases to form hydrides. However, the properties may vary depending on the type of intermolecular force that exists between the elements, their molecular masses, temperature, and other factors. Hydrides are mainly divided into three major types or groups. The categories are decided based on what elements the hydrogen forms bonds with or simply on the basis of chemical bonding.
Ahaana krishna upcoming movie
Sign in. They are white, high melting point solids that react immediately with protic solvents, for example:. They do conduct heat and electricity but not to the extent of their parent metals. Hydrides are chemical compounds with one atom of hydrogen and an extra atom. Hyrogen anion reacts with less electronegative elements to form compounds called hydrides. Yes, NH 3 or ammonia is a hydride of Nitrogen. The hydride formula of any compound is, MH x where M is less electronegative element and x is the oxidation number of M. Hydrides are classified into three major groups, depending on what elements the hydrogen bonds to. Ammonium chloride is widely used in dry-cell batteries and clean metals. Covalent hydrides are formed when hydrogen reacts with other similar electronegative elements like Si, C, etc. They bond with more electropositive metal atoms. Test Your Knowledge On Hydrides!
Hydride in Chemistry is the name of a compound containing Hydrogen Anion.
What occur in these hydrogen bonds are strong dipole-dipole attractions because of the high ionic character of the compounds. Thus, hydride hydrogen anion has a negative charge. Prateek Sharma 7. One interesting and unique characteristic of these hydrides are that they can be nonstoichiometric, meaning basically that the fraction of H atoms to the metals are not fixed. Saline hydrides are formed by the Group 1 and 2 metals when heated with dihydrogen H 2. Louis: Elsevier, Watch Now. They bond with more electropositive metal atoms. These compounds form between hydrogen and the most active metals, especially with the alkali and alkaline-earth metals of group one and two elements. You will be notified via email once the article is available for improvement.

Logically
In my opinion here someone has gone in cycles
I think, that you are mistaken. I can prove it. Write to me in PM, we will discuss.