How many electrons are in chlorine
Examples of Compounds with Chlorine Sodium Chloride This is sodium chloridealso known as table salt. Most people scientist know that the formula for salt is NaCl.
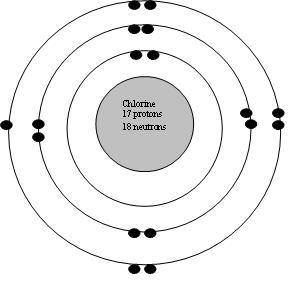
Additionally, a more basic way of determining the number of valence electrons would be to simply look at what group Cl is in. Chlorine has an atomic number Atomic number is the number of protons present in the nucleus of an atom. All the atoms of an element have same atomic number. In every stable atom the number of electrons is equal to the number of protons. Therefore, the number of electrons is equal to the number of protons in an chlorine atom.
How many electrons are in chlorine
Discover all the products made possible by chlorine chemistry through our chlorine and sodium hydroxide product trees. Chlorine Facts and Science. Chlorine is an element with unique properties Elemental chlorine gas Cl 2 is a yellow-green gas at room temperature and has a pungent odor similar to bleach even at very low concentrations. Chlorine has an atomic number of 17 and an atomic mass of As a member of the halogen family on the Periodic Table, chlorine is very reactive with metals and forms salts. The density of chlorine is The high density of chlorine gas causes it to sink if released into the ambient environment. In the ocean, chlorine is the third most common element, at a prevalence of 1. Overall, chlorine is the 23 rd most prevalent element in the universe. In nature, chlorine is found combined with other elements, such as in salt compounds, carnallite, and sylvite. Some volcanoes emit elemental chlorine gas Cl 2.
How many valence electrons are in an atom of chlorine?
Electron Configuration Notation: -shows the arrangment of electrons around the nucleus of an atom. How to Write the Electron Configuration for Chlorine Cl In order to write the Chlorine electron configuration we first need to know the number of electrons for the Cl atom there are 17 electrons. When we write the configuration we'll put all 17 electrons in orbitals around the nucleus of the Chlorine atom. In writing the electron configuration for Chlorine the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Chlorine go in the 2s orbital.
Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity, behind only oxygen and fluorine. While perhaps best known for its role in providing clean drinking water, chlorine chemistry also helps provide energy-efficient building materials, electronics, fiber optics, solar energy cells, 93 percent of life-saving pharmaceuticals, 86 percent of crop protection compounds, medical plastics, and much more. Elemental chlorine is commercially produced from brine by electrolysis, predominantly in the chlor-alkali process. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. For stable elements, there is usually a variety of stable isotopes. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons.
How many electrons are in chlorine
Electron Configuration Notation: -shows the arrangment of electrons around the nucleus of an atom. How to Write the Electron Configuration for Chlorine Cl In order to write the Chlorine electron configuration we first need to know the number of electrons for the Cl atom there are 17 electrons. When we write the configuration we'll put all 17 electrons in orbitals around the nucleus of the Chlorine atom. In writing the electron configuration for Chlorine the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Chlorine go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s.
Khatrimaza movie download bollywood
Chlorine has 7 valence electrons. Why are valence electrons responsible for the behavior of the atom? How many valence electrons are in carbon? A workhorse element with a wide range of important applications Below are some of the primary uses of chlorine chemistry: Swimming pool water—Kills germs in pool water to help control the spread of waterborne illnesses. Now, the last shell of chlorine atom has 7 electrons in it. Elemental chlorine gas Cl 2 is manufactured using the chlor-alkali process, which uses electrolysis to transform highly concentrated salt water brine into chlorine, sodium hydroxide, and hydrogen. The high density of chlorine gas causes it to sink if released into the ambient environment. Electron Configuration Notation: -shows the arrangment of electrons around the nucleus of an atom. Video from: Noel Pauller. Here, stable means that atom has not formed a ion yet. It is in Group 17, which means it has 7 valence electrons. They are paid advertisements and neither partners nor recommended web sites.
In fact, the table mentioned below is the perfect information box Which gives you every single detail about the Chlorine element in Periodic table.
Hydrochloric acid also has many uses including processing steel and food products like gelatin and sugar, and producing batteries. Paper—Used as an oxidizing and bleaching agent in the pulp and paper industry. Sodium is "happy" because it has now given up its one extra electron. Therefore, there are 7 valence electrons in an chlorine atom. Chlorine Facts and Science. Chlorine is an element with unique properties Elemental chlorine gas Cl 2 is a yellow-green gas at room temperature and has a pungent odor similar to bleach even at very low concentrations. You can reuse this answer Creative Commons License. Now, the last shell of chlorine atom has 7 electrons in it. Each of the chlorine atoms gets an electron to fill its shell, and the aluminum loses three, giving it a filled shell too remember, Aluminum has three extra electrons. In a picture, the valence electrons are the ones in the outermost shell. Here, stable means that atom has not formed a ion yet.

This brilliant idea is necessary just by the way
It is a pity, that now I can not express - there is no free time. But I will be released - I will necessarily write that I think on this question.
Thanks for council how I can thank you?