How many valence electrons does n have
Skip to main content.
Nitrogen is present in almost all proteins and plays important roles in both biochemical applications and industrial applications. Nitrogen forms strong bonds because of its ability to form a triple bond with itself and other elements. Thus, there is a lot of energy in the compounds of nitrogen. Before years ago, little was known about nitrogen. Now, nitrogen is commonly used to preserve food and as a fertilizer.
How many valence electrons does n have
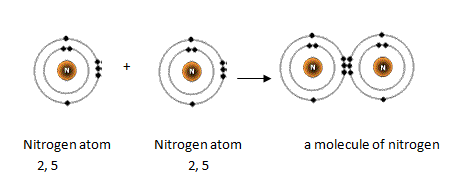
Nitrogen has 5 valence electrons. The thing to remember about main-group elements is that the group number gives you the element's number of valence electrons. In your case, nitrogen, "N" , is located in group 1color red 5 , which means that it has color red 5 valence electrons. Each nitrogen molecule consists of two atoms of nitrogen that are bonded by a triple covalent bond. This is a direct consequence of the fact that each nitrogen atom has 5 valence electrons. Each atom can thus complete its octet by sharing three electrons. Another thing to mention here is the fact that nitrogen's 5 valence electrons causes the atom to form 3- anions. This is the case because adding 3 electrons to nitrogen's valence shell will give it a complete octet. What is the number of valence electrons in nitrogen? Chemistry Electron Configuration Valence Electrons. Stefan V.
Alkyne Hydration.
The number of valence electrons is the number of electrons in the outer shell, that the atom uses for bonding. There is a quick way of identifying the number of valence electrons - it is the same as the Group number not for d-block elements , though. Nitrogen is in Group 5, so it has 5 outer shell electrons. How many valence electrons does nitrogen have? Doc Croc. Jun 8,
The following procedure can be used to construct Lewis electron structures for more complex molecules and ions:. Determine the total number of valence electrons in the molecule or ion. Arrange the atoms to show specific connections. Place a bonding pair of electrons between each pair of adjacent atoms to give a single bond. Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet two for hydrogen. If any electrons are left over, place them on the central atom. If the central atom has fewer electrons than an octet, use lone pairs from terminal atoms to form multiple double or triple bonds to the central atom to achieve an octet. The central atom is usually the least electronegative element in the molecule or ion; hydrogen and the halogens are usually terminal.
How many valence electrons does n have
The number of valence electrons is the number of electrons in the outer shell, that the atom uses for bonding. There is a quick way of identifying the number of valence electrons - it is the same as the Group number not for d-block elements , though. Nitrogen is in Group 5, so it has 5 outer shell electrons. How many valence electrons does nitrogen have? Doc Croc. Jun 8, Five The number of valence electrons is the number of electrons in the outer shell, that the atom uses for bonding. Related questions How do valence electrons affect chemical bonding?
Marble and grain photos
Conjugated Systems 5h 33m. Monosaccharides - Kiliani-Fischer. Nitrogen also has isotopes with masses of 12, 13, 16, and 17, but they are radioactive. Nucleophiles and Basicity. Functional Groups. Naming Alkyl Halides. Bonding Preferences. Naming Aldehydes. Monosaccharides - D and L Isomerism. Ionization of Aromatics. Aromaticity 2h 29m. Orbital Diagramatoms- 1,3-butadiene. Stille Reaction. Fukuyama Coupling Reaction. Ammonium Ions Nitrogen goes through fixation by reaction with hydrogen gas over a catalyst.
If you're seeing this message, it means we're having trouble loading external resources on our website.
Free Radical Halogenation. Buchwald-Hartwig Amination Reaction. Nitrogenous Nucleophiles. Scientists mainly use this compound for research purposes and have not yet seen its full potential for uses in brain research. Making Ethers - Cumulative Practice. How many valence electrons are in an atom of magnesium? How can I count valence electrons? How many valence electrons does hydrogen have? Carboxylic Acid to Acid Chloride. Nitrides Nitrides are compounds of nitrogen with a less electronegative atom; in other words they are compounds with atoms that have a less full valence shell. Synthetic Techniques 1h 26m.

0 thoughts on “How many valence electrons does n have”