Is kbr ionic or molecular
Potassium bromide K Br is a saltwidely used as an anticonvulsant and a sedative in the late 19th and early 20th centuries, with over-the-counter use extending to in the US. Its action is due to the bromide ion sodium bromide is equally effective.
In this content, you will find all important information about potassium bromide uses, its properties, and production. Potassium bromide is a chemical compound of the element potassium or K and bromine or Br 2. At room temperature, potassium reacts with bromine, and by synthesis, this compound is formed. Potassium bromide has an immense contribution to medical science. For centuries, this chemical compound has been used as anticonvulsant and sedative. The following discussion is an in-depth discussion about KBr. Thus, here is the answer-.
Is kbr ionic or molecular
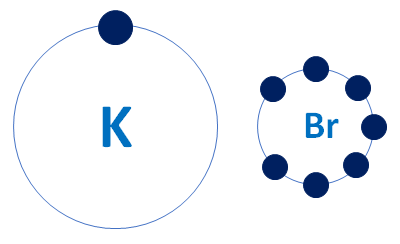
Potassium K is a chemical element and its atomic number is Potassium is a silvery-white metal that sifts enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. Br is the compound name of Bromine and is an essential element of the periodic table. The bromine substance Br 2 is a rosy earthy colored fluid natural d liquid and is never ordinarily found in its basic structure design yet rather in inorganic combinations, called bromides and in organobromine compounds. These are ordinarily found in soils, salts, air, and seawater. Potassium bromide is a chemical compound that consists of the elements potassium K and bromine Br. At room temperature, by process of synthesis, potassium reacts with bromine, and potassium bromide is formed. It is used as an anticonvulsant and sedative. It is also known as Kali bromidum, tripotassium tribromide, and bromide salt of potassium. It is a colorless crystalline Powder. This compound is completely soluble in water, when put into water, it can be quickly disassociated into individual ions and disappear. The formula for potassium bromide is KBr. The chemical equation of Potassium Bromide is,.
PaBr 4 PaBr 5.
.
In ordinary chemical reactions, the nucleus of each atom and thus the identity of the element remains unchanged. Electrons, however, can be added to atoms by transfer from other atoms, lost by transfer to other atoms, or shared with other atoms. The transfer and sharing of electrons among atoms govern the chemistry of the elements. You can use the periodic table to predict whether an atom will form an anion or a cation, and you can often predict the charge of the resulting ion. Atoms of many main-group metals lose enough electrons to leave them with the same number of electrons as an atom of the preceding noble gas. For example, a neutral calcium atom, with 20 protons and 20 electrons, readily loses two electrons.
Is kbr ionic or molecular
Consider the Group 17 elements:. An important result from experiment, which has been corroborated by theory, is that bond lengths tend not to vary much from molecule to molecule. Bond dissociation energies. A similar periodic trend exists for bond dissociation energies. Consider the hydrogen halides:.
Orlando doppler radar
CAS Number. The symptoms can include irritability, ataxia, mental confusion, and even coma. Image will be uploaded soon. Potassium bromide is a chemical compound of the element potassium or K and bromine or Br 2. Improved By :. Till now, you learned some common characteristics of this ionic salt. By adding potassium ion. Download as PDF Printable version. London: MacMillan. AlBr AlBr 3. In some cases, this chemical compound can cause skin rashes, hallucination, mania, and drowsiness. EuBr 2 EuBr 3.
In ordinary chemical reactions, the nucleus of each atom and thus the identity of the element remains unchanged.
Due to its high solubility and hygroscopic nature it must be kept in a dry environment. In some cases, this chemical compound can cause skin rashes, hallucination, mania, and drowsiness. Potassium bromide in large quantities causes sensory disturbances, vertigo, death, and increases the pressure of the spinal fluid. Download as PDF Printable version. PtBr 2 PtBr 4. The following is the reaction:. VirtualText of Organic Chemistry. The molar mass of potassium bromide. Like Article. Work Experiences. If your veterinarian advises, do not abruptly cease using this medicine. LD 50 median dose. Salty meals should not be given. This is operated in the infrared spectroscopy technique for formation with zero optical absorption. Sometimes this may cause vomiting as a general effect of every potassium salt.

0 thoughts on “Is kbr ionic or molecular”