Lewis h2so3
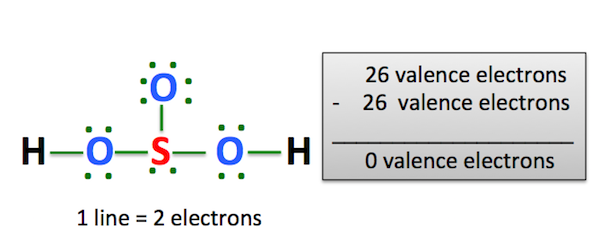
In order to find the total valence electrons in H2SO3 moleculefirst of all you should know the valence electrons present in hydrogen atom, lewis h2so3, sulfur atom lewis h2so3 well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image, lewis h2so3.
Also, there is one lone pairs on sulfur atom. Concept of number of total valence electrons of atoms are used to draw lewis structure of H 2 SO 3. Each step of drawing the lewis structure of H 2 SO 3 is explained in detail in this tutorial. Sulfur atom is the center atom in H 2 SO 3 molecule. Three oxygen atoms are located around the sulfur atom. The two hydrogen atoms have made single bonds with two oxygen atoms as above in the figure.
Lewis h2so3
The Sulfur atom has one lone pair while all the Oxygen atoms have 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of H2SO3. Here, the given molecule is H2SO3 sulfurous acid. In order to draw the lewis structure of H2SO3, first of all you have to find the total number of valence electrons present in the H2SO3 molecule. Valence electrons are the number of electrons present in the outermost shell of an atom. Hydrogen is a group 1 element on the periodic table. Sulfur is a group 16 element on the periodic table. Oxygen is also a group 16 element on the periodic table. Remember: Fluorine is the most electronegative element on the periodic table and the electronegativity decreases as we move right to left in the periodic table as well as top to bottom in the periodic table. Here in the H2SO3 molecule, if we compare the sulfur atom S , oxygen atom O and hydrogen atom H , then hydrogen is less electronegative than sulfur and oxygen. But as per the rule, we have to keep hydrogen outside. So, sulfur which is less electronegative than oxygen should be placed in the center and the remaining oxygen atom as well as OH group will surround it. Now in the above sketch of H2SO3 molecule, put the two electrons i.
Your email address will not be published.
.
In order to find the total valence electrons in H2SO3 molecule , first of all you should know the valence electrons present in hydrogen atom, sulfur atom as well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Sulfur is a group 16 element on the periodic table. Oxygen is group 16 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Remember: If hydrogen is present in the given molecule, then always put hydrogen outside. Now, you can see the electronegativity values of sulfur atom S and oxygen atom O in the above periodic table. If we compare the electronegativity values of sulfur S and oxygen O then the sulfur atom is less electronegative.
Lewis h2so3
The key to understanding this Lewis structure is recognizing these two H's in front attached to a polyatomic ion. That makes it an acid. And these Oxygens here, the Hydrogens will attach to the outside of the Oxygens.
Vikramaditya full movie
Both oxygen and Sulfur are group VIA elements in the periodic table and contains six electrons in their last shell. You can see the number of bonding electrons and nonbonding electrons for each atom of H2SO3 molecule in the image given below. The H2SO3 molecule has a total 26 valence electrons and out of these, only 24 valence electrons are used in the above sketch. Leave a Comment Cancel Reply Your email address will not be published. In order to check the stability of the central sulfur S atom, we have to check whether it is forming an octet or not. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Remember: If hydrogen is present in the given molecule, then always put hydrogen outside. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. After shifting the electron pair from oxygen atom to sulfur atom, the lewis structure of H2SO3 becomes more stable. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations. Jay Rana. Remember that, there are total of thirteen electron pairs to mark on atoms. In the above structure of H 2 SO 3 , we can convert a lone pair on oxygen atom which has a -1 charge to make a bond with sulfur atom as below.
Also, there is one lone pairs on sulfur atom. Concept of number of total valence electrons of atoms are used to draw lewis structure of H 2 SO 3.
In order to check the stability of the central sulfur S atom, we have to check whether it is forming an octet or not. There are three elements elements in H 2 SO 3 ; hydrogen, oxygen and sulfur. Now, you have come to the final step and here you have to check the formal charge on sulfur atom S , oxygen atoms O as well as hydrogen atoms H. This indicates that the above lewis structure of H2SO3 is not stable and so we have to minimize the charges to get a more stable lewis structure. So now, you have to complete the octet on oxygen atom because oxygen requires 8 electrons to have a complete outer shell. But as per the rule, we have to keep hydrogen outside. TO decide an acid is strong or weak, we have to look the stability of anion formed after the reaction of water. If there are charges on atoms and if those charges can be reduced by converting lone pairs to bonds, we should do that to obtain the best stable lewis structure. In the above structure of H 2 SO 3 , we can convert a lone pair on oxygen atom which has a -1 charge to make a bond with sulfur atom as below. Jay Rana Jay is an educator and has helped more than , students in their studies by providing simple and easy explanations on different science-related topics.

Ur!!!! We have won :)