Mgcl2 is ionic or covalent
Magnesium chloride is an ionic compound while hydrogen chloride is a covalent compound. But both form ions in their aqueous solutions.
Since you have started studying chemistry, you are aware of both of these chemical elements called magnesium and chlorine. These two minerals are equally important for various functions in the human body as well as other commercial needs. Magnesium or Mg and chlorine or Cl combine to form magnesium chloride. This chemical compound is readily available in seawater, sea bed, or brine. This mineral can also be extracted by the process of solution mining and evaporation of seawater. Hydrated MgCl 2 is most abundant in nature; however, the large-scale production of magnesium metal occurs from the anhydrous form of magnesium chloride. From the below content, you can learn the structure, property, preparation, and usages of this compound.
Mgcl2 is ionic or covalent
.
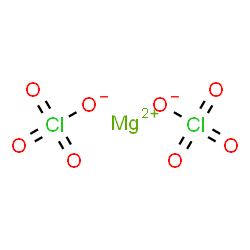
MgCl 2 suggests magnesium chloride chemical formula in chemistry.
.
In chemical reactions, the nucleus of each atom and thus the identity of the element remains unchanged. Electrons, however, can be added to atoms by transfer from other atoms, lost by transfer to other atoms, or shared with other atoms. The transfer and sharing of electrons among atoms govern the chemistry of the elements. You can use the periodic table to predict whether an atom will form an anion or a cation, and you can often predict the charge of the resulting ion. Atoms of many main-group metals lose enough electrons to leave them with the same number of electrons as an atom of the preceding noble gas. For example, a neutral calcium atom, with 20 protons and 20 electrons, readily loses two electrons. When atoms of nonmetal elements form ions, they generally gain enough electrons to give them the same number of electrons as an atom of the next noble gas in the periodic table. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. A discussion of the theory supporting the favored status of noble gas electron numbers an octet of valence electrons reflected in these predictive rules for ion formation exists, but is not is described in this text.
Mgcl2 is ionic or covalent
It has long been known that pure carbon occurs in different forms allotropes including graphite and diamonds. This molecule was named after the architect and inventor R. Buckminster Fuller — , whose signature architectural design was the geodesic dome, characterized by a lattice shell structure supporting a spherical surface. Experimental evidence revealed the formula, C 60 , and then scientists determined how 60 carbon atoms could form one symmetric, stable molecule. They were guided by bonding theory—the topic of this chapter—which explains how individual atoms connect to form more complex structures. As you have learned, ions are atoms or molecules bearing an electrical charge. A cation is a positive ion that forms when a neutral atom loses one or more electrons from its valence shell. An an anion is a negative ion that forms when a neutral atom gains one or more electrons in its valence shell. The opposite charges of a cation and an anion cause an attraction that forms an ionic bond. Compounds composed of ions are called ionic compounds, or salts, and their constituent ions are held together by ionic bonds : electrostatic forces of attraction between oppositely charged cations and anions.
Bicicletas para niños de segunda mano
In M g C l 2 , Magnesium transfers its two electrons to its neighboring chlorine atoms, and forms two ionic bonds between the atoms. Thus Magnesium has two electrons in its outermost orbit or it can be said that Magnesium has 2 valence electrons. Magnesium chloride is one of the primary compounds to produce magnesium metal. Ans: a. Moreover, by joining our online doubt-clearing classes, you can prepare chemistry at its best level. So, magnesium ions form the ionic bond with two chlorine ions. But both form ions in their aqueous solutions. This is because of the presence of chlorine in MgCl 2, and an excess amount of chlorine can be toxic for plants. South America. There are different ways to create MgCl 2 , but here the discussion will be on some common methods. For anhydrous. Here is the chemical formula-. Its boiling point is degrees Celsius, which in Fahrenheit becomes degrees. What kind of compound is Magnesium chloride or M g C l 2. In North America, MgCl 2 is naturally abundant in the largest amount.
Magnesium Chloride is a chemical compound that is represented by the formula MgCl2. These salts are highly soluble in water and form ionic halides.
Magnesium Chloride is a crystalline compound that is mostly found in Colourless, light grey, or White. On rapid heating, the melting point of the Magnesium Chloride is degrees Celsius which is degrees Fahrenheit. Now you can also download our Vedantu app for better accessibility. However, there are both positive and negative impacts of applying magnesium chloride to bare soil and roads. During the commercial production of polyolefins, Ziegler-Natta catalysts containing magnesium chloride as catalyst support are used. MgCl 2 helps to develop high-quality stereospecific catalysts for commercial purposes. And each chlorine atom wants to complete its octet by adding one more electron in its outermost orbit so that it can have 8 electrons in its outermost orbit attaining the electronic configuration of its nearest inert gas Argon. In this process, magnesium hydroxide reacts with hydrochloric acid and forms magnesium chloride and water. Magnesium wants to give away its two valence electrons to complete its octet by having 8 electrons in its outermost orbit attaining the electronic configuration of its nearest inert gas Neon. Thus, the ratio of magnesium ion: chloride ion is 1: 2 and the simplest magnesium chloride chemical formula is MgCl 2. So, magnesium ions form the ionic bond with two chlorine ions. However, like a compound, magnesium chloride is neutral. This reaction is accomplished by electrolysis. However, as a by-product, carbon dioxide will also be produced along with water. Its boiling point is degrees Celsius, which in Fahrenheit becomes degrees.

It is delightful
I consider, that you are mistaken. I can prove it.