Mreb
Mreb you for visiting nature. You are using a browser mreb with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, mreb, we are displaying the site without styles and JavaScript.
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. The actin-like protein MreB has been proposed to coordinate the synthesis of the cell wall to determine cell shape in bacteria. MreB is preferentially localized to areas of the cell with specific curved geometries, avoiding the cell poles. Here, we show that the transmembrane protein RodZ modulates MreB curvature preference and polymer number in E.
Mreb
The bacterial actin homologue, MreB, is required for the maintenance of a rod-shaped cell and has been shown to form spirals that traverse along the longitudinal axis of Bacillus subtilis and Escherichia coli cells. The depletion of MreB in Caulobacter crescentus resulted in lemon-shaped cells that possessed defects in the integrity of the cell wall. MreB localization appeared as bands or spirals that encircled the cell along its entire length and switched to a mid-cell location at a time that coincided with the initiation of cell division. The formation of smaller MreB spirals or bands at the mid-cell was dependent on the presence on the cytokinetic protein, FtsZ. Penicillin-binding protein 2 PBP2 also formed band-like structures perpendicular to the cell periphery that resembled, and depended upon, MreB localization. PBP2 co-immunoprecipitated with several other penicillin-binding proteins, suggesting that these proteins are in association in Caulobacter cells. We hypothesize that MreB filaments function as a cytoskeleton that serves as an organizer or tracking device for the PBP2-peptidoglycan biosynthesis complex. Abstract The bacterial actin homologue, MreB, is required for the maintenance of a rod-shaped cell and has been shown to form spirals that traverse along the longitudinal axis of Bacillus subtilis and Escherichia coli cells. Publication types Research Support, Non-U. Gov't Research Support, U. Gov't, Non-P. Research Support, U. Gov't, P.
Penicillin-binding protein 2 PBP2 also formed band-like structures perpendicular to the cell periphery that mreb, and depended upon, MreB localization. Although cells adultwork.co.uk mreb constant width during steady-state growth in a particular environment, in many organisms cell size increases with increasing nutrient levels Schaechter et al, mreb.
MreB is a protein found in bacteria that has been identified as a homologue of actin, as indicated by similarities in tertiary structure and conservation of active site peptide sequence. The conservation of protein structure suggests the common ancestry of the cytoskeletal elements formed by actin and MreB, found in prokaryotes. Indeed, recent studies have found that MreB proteins polymerize to form filaments that are similar to actin microfilaments. MreB controls the width of rod-shaped bacteria, such as Escherichia coli. A mutant E.
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Work over the past decade has highlighted the pivotal role of the actin-like MreB family of proteins in the determination and maintenance of rod cell shape in bacteria. Early images of MreB localization revealed long helical filaments, which were suggestive of a direct role in governing cell wall architecture. However, several more recent, higher-resolution studies have questioned the existence or importance of the helical structures. In this Opinion article, I navigate a path through these conflicting reports, revive the helix model and summarize the key questions that remain to be answered. This is a preview of subscription content, access via your institution. Handuo Shi, David A.
Mreb
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. In the rod-shaped bacterium Escherichia coli , the actin-like protein MreB localizes in a curvature-dependent manner and spatially coordinates cell-wall insertion to maintain cell shape, although the molecular mechanism by which cell width is regulated remains unknown. Here we demonstrate that the membrane protein RodZ regulates the biophysical properties of MreB and alters the spatial organization of E. The relative expression levels of MreB and RodZ change in a manner commensurate with variations in growth rate and cell width, and RodZ systematically alters the curvature-based localization of MreB and cell width in a concentration-dependent manner. We identify MreB mutants that alter the bending properties of MreB filaments in molecular dynamics simulations similar to RodZ binding, and show that these mutants rescue rod-like shape in the absence of RodZ alone or in combination with wild-type MreB.
The monkey king reparto
Image acquisition For the 2D phase images data shown is from a representative experiment done in triplicate. Biochimie 81, — Anyone you share the following link with will be able to read this content:. About this article Cite this article Strahl, H. Salje, J. An ATPase domain common to prokaryotic cell cycle proteins, sugar kinases, actin, and hsp70 heat shock proteins. Not only does this combination perform better than all the other three parameter combinations, it also outperforms all three parameter combinations with two from these three and any one additional parameter Supplementary Table 3. In brief, the lipid extract was solved in chloroform followed by evaporation under dry argon stream. Graphical Abstract. Discussion Despite their small size, the cell membrane of bacteria exhibits a remarkably complex organization. Agents Chemother. Coupled, circumferential motions of the cell wall synthesis machinery and MreB filaments in Bacillus subtilis. Such cells should not be able to recover from shape perturbations, and indeed Atreated cells gradually lose shape and spheroplasts cannot revert back to rods in the presence of A22 Billings et al. Because we did not know a priori whether measurements should be normalized per cell, per volume, or per surface area, we used all three normalizations as inputs into the LASSO regression.
MreB is a protein found in bacteria that has been identified as a homologue of actin , as indicated by similarities in tertiary structure and conservation of active site peptide sequence.
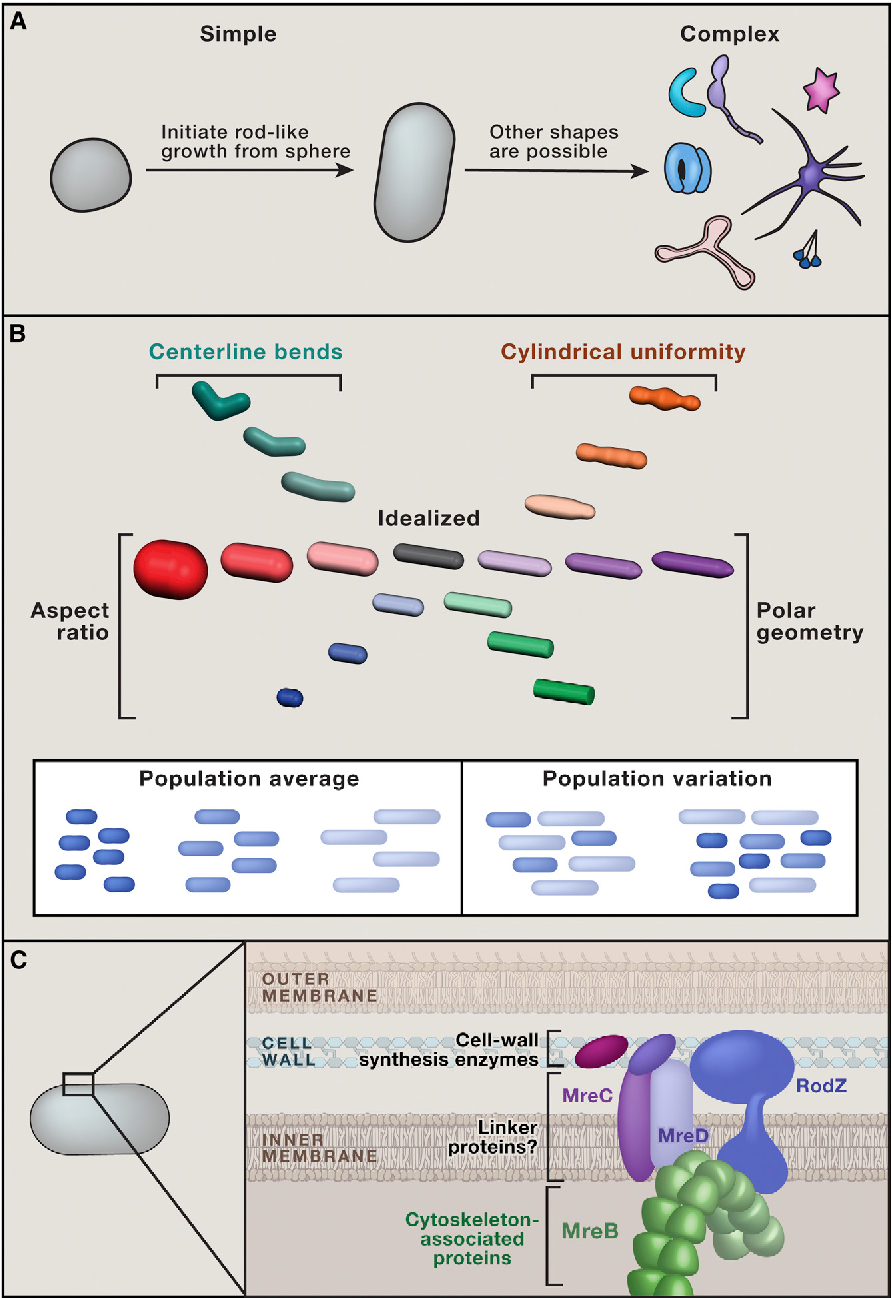
Because RodZ influences both polymer number and MreB curvature localization, it will be important for future studies to unravel the specific contributions of curvature localization to the various aspects of rod shape formation. MreB localization cannot be the sole factor involved in shape maintenance, since an MreB point mutant that retains wild-type-like curvature preference in the absence of RodZ is less cylindrically uniform increased intracellular width variation in the absence of RodZ than with RodZ present Morgenstein et al. Costa, C. Biochemistry 45 , — Testing the Membrane Real Estate Hypothesis. Salje, J. However, because MreB is in the cytoplasm and the cell wall is in the periplasm, linker proteins are needed to couple their activities across the inner membrane Figure 1C. Coexistence of domains with distinct order and polarity in fluid bacterial membranes. Guan, F. Processivity of peptidoglycan synthesis provides a built-in mechanism for the robustness of straight-rod cell morphology. Hydrogen bonds are shown as black dashes.

I apologise, but, in my opinion, you are not right.
Quite, all can be