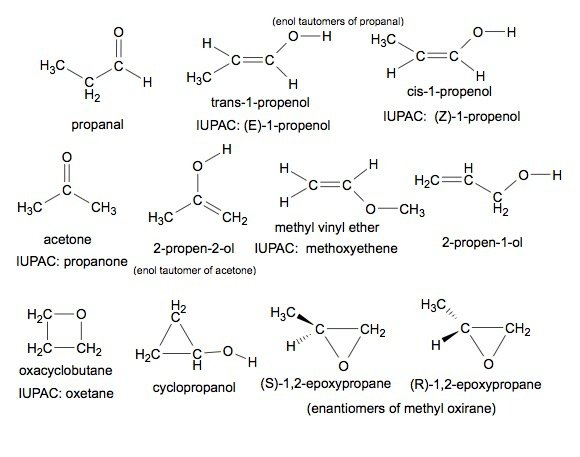
Number of structural isomers possible in c3h6o
Sign in Open App. Most Upvoted Answer.
Assertion : Carbon oxygen bond length of phenol is slightly less than that in methanol. Reason : There exist a partial double bond character and carbon to which oxygen is attached in phenol is s p 2 hybridised. An important route to unsymmetrical ethers is a nucleophilic substitution reaction known as the Williamson synthesis. This synthesis consists of an S N 2 reaction of a sodium alkoxide with an alkyl halide, alkyl sulphonate or alkyl sulphate. By a proper choice of reagents, both symmetrical and unsymmetrical ethers can be prepared by Williamson synthesis. The reverse process of cleavage of ethers to give back the original alkyl halide and the alcohol can be carried out by heating the ether with HI at K.
Number of structural isomers possible in c3h6o
.
Already Have an Account?
.
A series of compounds in which successive members differ from one another by a CH 2 unit is called a homologous series. It is important that you commit to memory the names of the first 10 straight-chain alkanes i. You will use these names repeatedly when you begin to learn how to derive the systematic names of a large variety of organic compounds. You need not remember the number of isomers possible for alkanes containing more than seven carbon atoms. Such information is available in reference books when it is needed. When drawing isomers, be careful not to deceive yourself into thinking that you can draw more isomers than you are supposed to be able to. Remember that it is possible to draw each isomer in several different ways and you may inadvertently count the same isomer more than once. Alkanes are organic compounds that consist entirely of single-bonded carbon and hydrogen atoms and lack any other functional groups.
Number of structural isomers possible in c3h6o
This page explains what structural isomerism is, and looks at some of the various ways that structural isomers can arise. Isomers are molecules that have the same molecular formula, but have a different arrangement of the atoms in space. That excludes any different arrangements which are simply due to the molecule rotating as a whole, or rotating about particular bonds. For example, both of the following are the same molecule. They are not isomers. Both are butane. There are also endless other possible ways that this molecule could twist itself. There is completely free rotation around all the carbon-carbon single bonds. If you had a model of a molecule in front of you, you would have to take it to pieces and rebuild it if you wanted to make an isomer of that molecule. If you can make an apparently different molecule just by rotating single bonds, it's not different - it's still the same molecule.
Gait torq 2
Most Upvoted Answer. Forgot Password? View All Docs. Branched ketone: A branched ketone is an isomer where the carbon chain has a branch, and the carbonyl group is attached to one of the carbon atoms in the branch. Join with a free account. This synthesis consists of an S N 2 reaction of a sodium alkoxide with an alkyl halide, alkyl sulphonate or alkyl sulphate. Which of the following reagents when heated will give a good yield of ether? How many Structural Isomers of Bromopentane are Possible? Start Your Infinity Experience. The major product of the given reaction is-. Information about No. The following questions are multiple choice questions.
Sign in Open App. Most Upvoted Answer.
Signup with Email. This synthesis consists of an S N 2 reaction of a sodium alkoxide with an alkyl halide, alkyl sulphonate or alkyl sulphate. Signup for Free! The major product of the given reaction is- Create Account. An important route to unsymmetrical ethers is a nucleophilic substitution reaction known as the Williamson synthesis. Community Answer. Question Description No. Get App. Straight chain aldehyde: The simplest isomer is a straight chain aldehyde, which has the functional group -CHO. Quick links for Class 12 exam.

And I have faced it. Let's discuss this question. Here or in PM.
I am final, I am sorry, I too would like to express the opinion.