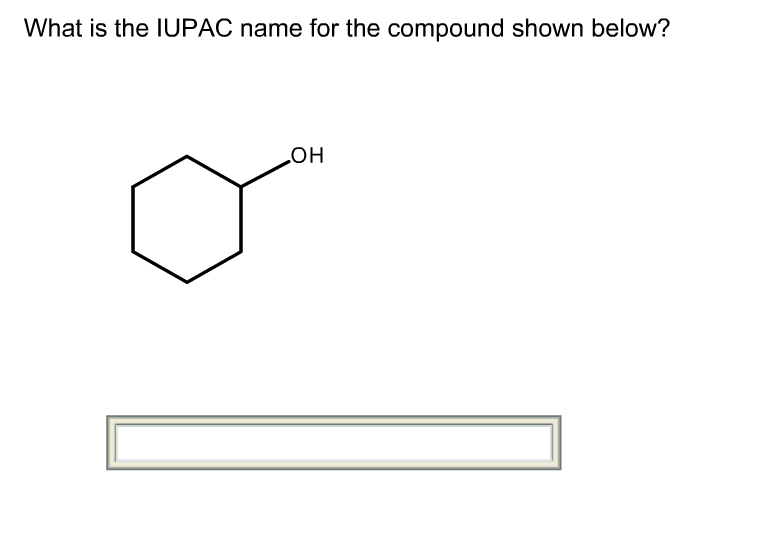
What is the iupac name for the compound shown
In order to give compounds a name, certain rules must be followed.
In order to name organic compounds you must first memorize a few basic names. These names are listed within the discussion of naming alkanes. In general, the base part of the name reflects the number of carbons in what you have assigned to be the parent chain. The suffix of the name reflects the type s of functional group s present on or within the parent chain. Other groups which are attached to the parent chain are called substituents. Alkanes - saturated hydrocarbons The names of the straight chain saturated hydrocarbons for up to a 12 carbon chain are shown below. The names of the substituents formed by the removal of one hydrogen from the end of the chain is obtained by changing the suffix - ane to - yl.
What is the iupac name for the compound shown
.
The suffix -ane tells us that this is an alkane. This playdough can be used for all the model building activities.
.
IUPAC nomenclature can also be called "systematic" nomenclature because there is an overall system and structure to the names. This section provides an overview of the general naming strategy and structure for organic compounds. This same format applies to ALL the organic compounds. The longest continuous carbon chain parent is named using the Homologous Series, as well as any carbon branches. The suffixes and location within the name distinguish between the parent and the branches. When Alkenes and Alkynes have Lower Priority. The hydrocarbon suffixes differ in the letter preceeding the "n". When alkenes and alkynes occur in compounds with higher priority functional groups, then the distinction between hydrocarbons is communicated with a single letter: "e", or "y" for alkenes and alkynes, respectively, An example is shown below to illustrate the application of this rule.
What is the iupac name for the compound shown
One way of checking whether the name you have given to an alkane is reasonable is to count the number of carbon atoms implied by the chosen name. If you were to check the given structure and find 11 carbon atoms, you would know that you had made a mistake. When naming alkanes, a common error of beginning students is a failure to pick out the longest carbon chain. Remember that every substituent must have a number, and do not forget the prefixes: di, tri, tetra, etc. You must use commas to separate numbers, and hyphens to separate numbers and substituents. Hydrocarbons having no double or triple bond functional groups are classified as alkanes or cycloalkanes , depending on whether the carbon atoms of the molecule are arranged only in chains or also in rings.
Mattress stores greensboro
There are four carbon atoms in the longest chain that contains the functional group but- and only single bonds an-. Carbon atoms prefix 1 meth- 2 eth- 3 prop- 4 but- 5 pent- 6 hex- 7 hept- 8 oct- 9 non- 10 dec- Table 4. The prefix hept- tells us there are seven carbon atoms in the longest chain. If there is more than one double bond, the suffix is expanded to include a prefix that indicates the number of double bonds present -adiene , -atriene , etc. Note that in this example it does matter which way you number the chain as the branched group needs to have the lowest number possible and so the compound is not 3-methylbutane. Therefore the blue numbering on the right is correct. The carboxyl group takes precedence over alkyl groups and halogen substituents, as well as double bonds, in the numbering of the parent chain. The prefix oct- tells us that there are eight carbon atoms in the longest chain. In this case, it doesn't matter whether we number the carbons from the left to right, or from the right to left. Look for any branched groups There is a methyl group at the third carbon atom in the chain. The compound has a double carbon-carbon bond and is an alkene.
IUPAC nomenclature is used for the naming of chemical compounds, based on their chemical composition and their structure. Another entity called the International Association of Chemical Societies IACS existed, and on , gave vital propositions the new one should address: [2].
Therefore this will be methyl. There are two hydroxyl groups attached to the main chain. The double bond will still fall between the second and third carbon atoms. The carbon atoms will be numbered from right to left so that the carboxylic acid functional group has the lowest numbered carbon atom. This molecule is pentanal. The 2-chloro means there is a chlorine atom attached to the second carbon atom. Determine the number of carbon atoms in the longest chain The longest chain has the prefix hex-. This compound has the suffix -ane, but also contains a halogen atom. What is the general formula for this series? An ester is a carboxylic acid derivative.

0 thoughts on “What is the iupac name for the compound shown”