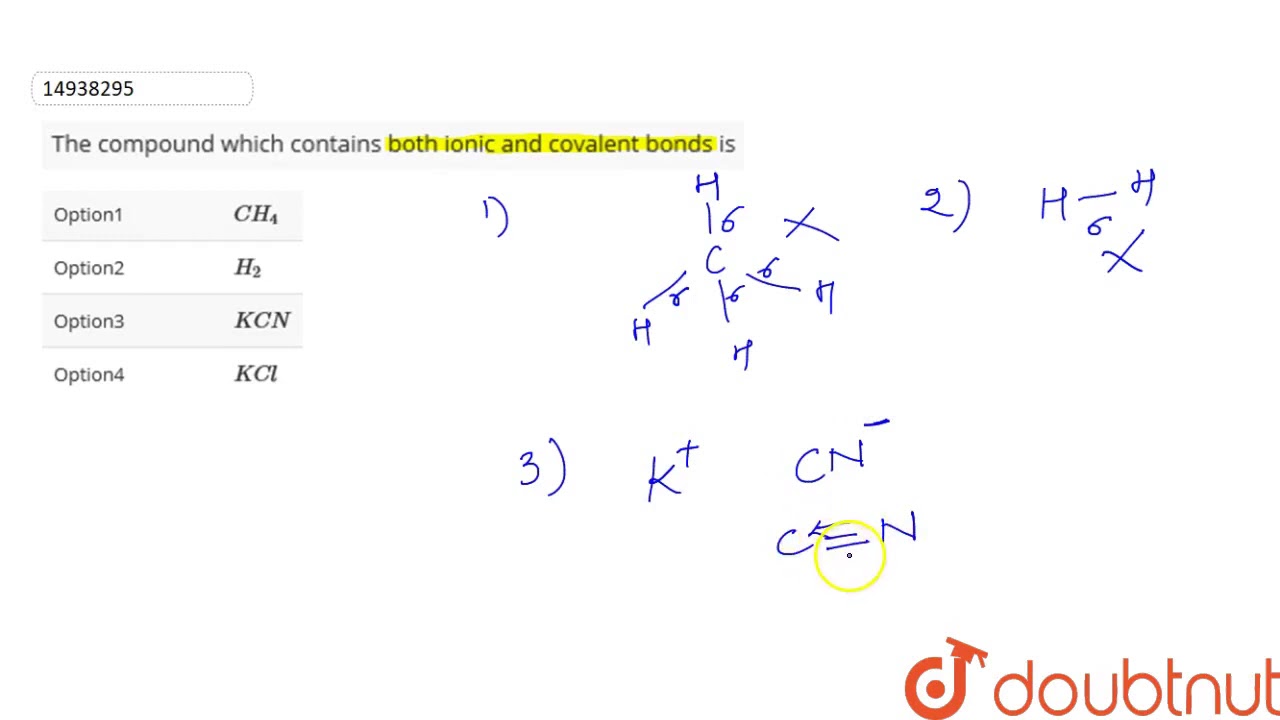
Compound containing both ionic and covalent bonds
An ionic bond is a chemical bond between two atoms in which one atom seems to donate its electron to another atom. Covalent bondson the other hand, appear to involve two atoms sharing electrons reach a more stable electron configuration. These compounds contain polyatomic ions.
Last updated on Jan 7, Get Started. SSC Exams. Banking Exams. Teaching Exams. Civil Services Exam. Railways Exams.
Compound containing both ionic and covalent bonds
Some chemical compounds contain both ionic and covalent bonds. These are ionic compounds that contain polyatomic ions. Often, a compound with both types of bonds contains a metal bonded to an anion of covalently bonded nonmetals. Less often, the cation is polyatomic. Sometimes nonmetals bond to form a cation with enough electronegativity difference from the anion to form an ionic bond! Here are examples of compounds with both ionic and covalent bonds. Remember, an ionic bond occurs when one atom essentially donates a valence electron to another atom. A covalent bond involves atoms sharing electrons. In pure covalent bonds, this sharing is equal. In polar covalent bonds, the electron spends more time with one atom than the other. For example, in potassium cyanide KCN , the carbon C and nitrogen N are both nonmetals, so they share a covalent bond. The potassium atom K is a metal, so it bonds to the nonmetallic anion via an ionic bond. X-ray diffraction of KCN crystals verifies this arrangement.
Understand audiences through statistics or combinations of data from different sources. NVS Electrician. BPSC Exam.
.
An ionic bond is a chemical bond between two atoms in which one atom seems to donate its electron to another atom. Covalent bonds , on the other hand, appear to involve two atoms sharing electrons reach a more stable electron configuration. These compounds contain polyatomic ions. Many of these compounds contain a metal, a nonmetal, and also hydrogen. However, other examples contain a metal joined via an ionic bond to covalently bonded nonmetals. Here are examples of compounds that exhibit both types of chemical bonding:. In ammonium sulfide, the ammonium cation and the sulfide anion are ionically bonded together, even though all of the atoms are nonmetals.
Compound containing both ionic and covalent bonds
As you have learned, ions are atoms or molecules bearing an electrical charge. A cation a positive ion forms when a neutral atom loses one or more electrons from its valence shell, and an anion a negative ion forms when a neutral atom gains one or more electrons in its valence shell. Compounds composed of ions are called ionic compounds or salts. The ions are held together by ionic bonds: electrostatic forces of attraction between oppositely charged cations and anions. The properties of ionic compounds shed some light on the nature of ionic bonds. Ionic solids exhibit a crystalline structure and tend to be rigid and brittle. They also tend to have high melting and boiling points, which suggests that ionic bonds are very strong. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely in the solid state.
John deere f680
Assam Rifles. Kerala Police Constable. Indian Coast Guard Yantrik. IBPS Clerk. Rajasthan High Court. Rajasthan High Court Civil Judge. Bihar Primary Teacher. BOB Acquisition Officer. Maharashtra Arogya Vibhag Group C. Telangana High Court Process Server. Trusted by 3. Assam TET.
In ordinary chemical reactions, the nucleus of each atom and thus the identity of the element remains unchanged.
These choices will be signaled to our partners and will not affect browsing data. BPSC Exam. NVS Group B. Maharashtra Adivasi Vikas Vibhag. Airforce Group X. Mazagon Dock Shipbuilders Non Executive. Remember, an ionic bond occurs when one atom essentially donates a valence electron to another atom. BEL Trainee Engineer. CG Vyapam Hostel Warden. Chandigarh Police Constable. DHS Assam Grade 3. UP Police. Learn today! CISF Fireman.

Now all became clear, many thanks for an explanation.
Same a urbanization any
Excuse for that I interfere � I understand this question. I invite to discussion.