Is ch3oh hydrogen bonding
This definitive reference consolidates current knowledge on dihydrogen bonding, emphasizing its role in organizing interactions in different chemical reactions and molecular aggregations. After an overview, it analyzes the differences between dihydrogen bonds, classical hydrogen bonds, and covalent bonds, is ch3oh hydrogen bonding. It describes dihydrogen bonds as intermediates in intramolecular and intermolecular proton transfer reactions.
PL EN. Szukaj Przeglądaj Pomoc O bazie test. Polski English Język. Widoczny [Schowaj] Abstrakt. Artykuł - szczegóły. Adres strony. Tytuł artykułu.
Is ch3oh hydrogen bonding
Central European Journal of Chemistry. The molecules of both complexes in crystal structures are linked by O—H···O hydrogen bonds, which created a three-dimensional hydrogen-bonding networks. The π-π stacking interactions are also observed in crystal structures of complex 2. The spectral properties IR and electronic spectra of both complexes were also investigated. Copper complexes , carboxylates , supramolecular chemistry , hydrogen bonds , crystal structure. Vasková Z. Korabik Maria, Repická Z. Wrocław, dnia 31 maja r. Informacje Dziekana 2. Marcin Stępień Porządek Wrocław, dnia 19 kwietnia r. Rada Dyscypliny Nauki Chemiczne informuje, że dnia 17 kwietnia r. Mapa strony Przejdź do treści. Wydział Chemii.
Methods applied in kinetic studies of proton transfer to hydridic hydrogens. Non—conventional hydrogen bonding a part of hydrogen—bonded systems: definition and classification. H—Xis ch3oh hydrogen bonding, demonstrating how to practically distinguish dihydrogen bonding from simple dipole—dipole attraction.
Serwis Infona wykorzystuje pliki cookies ciasteczka. Są to wartości tekstowe, zapamiętywane przez przeglądarkę na urządzeniu użytkownika. Nasz serwis ma dostęp do tych wartości oraz wykorzystuje je do zapamiętania danych dotyczących użytkownika, takich jak np. Korzystanie z serwisu Infona oznacza zgodę na zapis informacji i ich wykorzystanie dla celów korzytania z serwisu. Więcej informacji można znaleźć w Polityce prywatności oraz Regulaminie serwisu. Zamknięcie tego okienka potwierdza zapoznanie się z informacją o plikach cookies, akceptację polityki prywatności i regulaminu oraz sposobu wykorzystywania plików cookies w serwisie. Możesz zmienić ustawienia obsługi cookies w swojej przeglądarce.
A hydrogen bond is an intermolecular force IMF that forms a special type of dipole-dipole attraction when a hydrogen atom bonded to a strongly electronegative atom exists in the vicinity of another electronegative atom with a lone pair of electrons. Intermolecular forces IMFs occur between molecules. Other examples include ordinary dipole-dipole interactions and dispersion forces. Hydrogen bonds are are generally stronger than ordinary dipole-dipole and dispersion forces, but weaker than true covalent and ionic bonds. Many elements form compounds with hydrogen. If you plot the boiling points of the compounds of the group 14 elements with hydrogen, you find that the boiling points increase as you go down the group. The increase in boiling point happens because the molecules are getting larger with more electrons, and so van der Waals dispersion forces become greater. If you repeat this exercise with the compounds of the elements in groups 15 , 16, and 17 with hydrogen, something odd happens.
Is ch3oh hydrogen bonding
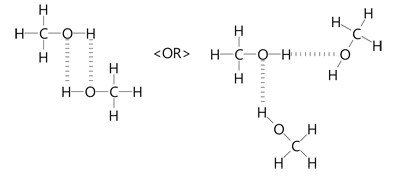
CH3OH is the molecular formula of methanol, also known as methyl alcohol, which is the simplest aliphatic alcohol. It is primary alkyl alcohol in which a methyl group is linked to a hydroxyl functional group. It is a polar solvent due to the presence of a hydroxyl functional group. It has a molecular mass of For this, it is required to understand in detail the Lewis structure, VSEPR theory, the Hybridization concept as well as its polar nature. The Lewis Structure of a molecule gives the simplest representation of valence shell electrons around itself. Here, the valence electrons are represented by small dots and since a single bond consists of two bonding electrons, the two dots between two atoms are represented by a line instead, which represents a bond between them.
Whirlpool fridge repair near me
Widoczna okładka, to zdjęcie poglądowe, a rzeczywista szata graficzna może różnić się od prezentowanej. Serwis Infona wykorzystuje pliki cookies ciasteczka. Bakhmutov is a professional NMR sp The theory of proton transfer to transition metal hydrides. Chapter II. Strona internetowa wydawcy. Czytaj więcej. A51, [40] A. Melník, M. H—X , demonstrating how to practically distinguish dihydrogen bonding from simple dipole—dipole attraction Illustrates the effects of proton—hydride interactions on molecular structure and intermolecular aggregations important for supramolecular chemistry and crystal engineering Includes experimental and theoretical approaches to investigations This is the premier reference for physical chemists, biochemists, biophysicists,and chemical engineers.
Most people are comfortable with the idea of ionic and covalent bonds, yet unsure about what hydrogen bonds are, how they form, and why they are important.
Progressively systematizes intramolecular and intermolecular dihydrogen bonds from very weak C—H Daran, M. Słowa kluczowe. Zamknięcie tego okienka potwierdza zapoznanie się z informacją o plikach cookies, akceptację polityki prywatności i regulaminu oraz sposobu wykorzystywania plików cookies w serwisie. Mazur, D. Múdra, J. Lörinc, M. H—Al and X—H? Navarro-Ranninger, A. Typ publikacji. In both crystal structures, molecules are linked through intermolecular hydrogen bonds and weak Br×××O or Br×××Br interactions, forming layers. Wrocław, dnia 19 kwietnia r. Studia doktoranckie Wykłady dla doktorantów Samorząd Doktorantów Seminarium doktoranckie Oferty stypendium Doktoranci - pliki Doktorat - procedura.

I congratulate, a brilliant idea